��Ŀ����
����Ŀ��ij��ɫ��������п��ܺ���![]() ��CO��
��CO��![]() ��
��![]() ��
��![]() �е�һ�ֻ��֣����ν������´���
�е�һ�ֻ��֣����ν������´���![]() �ٶ�ÿ�δ�������Ӧ��ȫ
�ٶ�ÿ�δ�������Ӧ��ȫ![]() ��(1)ͨ����ʯ��ʱ�����������С��(2)ͨ�����ȵ�����ͭʱ�������Ϊ��ɫ��(3)ͨ����ˮ����ͭ��ĩʱ����ĩ��Ϊ��ɫ��(4)ͨ������ʯ��ˮʱ����Һ����ǡ��ɴ˿���ȷ��ԭ���������
��(1)ͨ����ʯ��ʱ�����������С��(2)ͨ�����ȵ�����ͭʱ�������Ϊ��ɫ��(3)ͨ����ˮ����ͭ��ĩʱ����ĩ��Ϊ��ɫ��(4)ͨ������ʯ��ˮʱ����Һ����ǡ��ɴ˿���ȷ��ԭ���������
A.һ������![]() ��
��![]() �����ܺ���
�����ܺ���![]() ��CO
��CO
B.һ������![]() ��CO�����ܺ���
��CO�����ܺ���![]() ��
��![]()
C.һ������CO��![]() �����ܺ���
�����ܺ���![]() ��
��![]()
D.һ������CO��![]() �����ٺ���
�����ٺ���![]() ��
��![]() ��һ��
��һ��
���𰸡�D
��������
��ͨ����ʯ��ʱ�����������С����ʯ������ˮ�Ͷ�����̼�������С֤������������һ�֣�����ͨ����ʯ�Һ�ȫ�����գ�
��ͨ�����ȵ�����ͭʱ�������Ϊ��ɫ��������CO��ԭ����ͭ��Ҳ������������ԭ����ͭ��Ҳ���������߶��У�
��ͨ����ɫ����ͭ��ĩʱ����ĩ��Ϊ��ɫ��֤����ˮ���ɣ����ⲿ��ˮ��Դ��������ԭ����ͭʱ���ɣ�����һ����������
��ͨ������ʯ��ˮʱ����Һ�����֤���ж�����̼����������̼��Դ��һ����̼��ԭ����ͭ������һ����CO��
����������һ������CO��H2�����ٺ���H2O��CO2�е�һ�֣�
��ѡ��D��

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�����Ŀ������ʵ���ܴﵽԤ��Ŀ����
ʵ������ | ʵ��Ŀ�� | |
A | ��ijδ֪��Һ�м��� | �����Ƿ� |
B | ��ijδ֪��Һ�м���NaOH���壬���ȣ��ڹܿ���ʪ�����ɫʯ����ֽ���� | �����Ƿ� |
C | ���ȷֱ��� | ̽�� |
D | ����ʢ��Ũ�����ͭ���Թ� | ̽��Ũ�������ˮ�� |
A.AB.BC.CD.D
����Ŀ��ijѧ����0.2000mol/L�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ���¼�����
��������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ�� �۵���Һ������0������0���̶������£������¶���
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ
���ñ�Һ�ζ����յ㣬���µζ���Һ������� ��ش�
�����ϲ����д������(����)______���ô�������ᵼ�²ⶨ���(����ƫ��������ƫС��������Ӱ����)________
������ѡ����²ⶨ���ƫС����___________
A.������У��ڼ��µζ���Һ�����ʱ���ζ��ܼ���������
B.�ζ����յ����ʱ�����ӵζ��ܵĿ̶ȣ�����������ȷ
C.ʢװδ֪Һ����ƿ������ˮϴ��������δ֪Һ��ϴ
D.�ζ����յ����ʱ�����ֵζ��ܼ��촦����һ����Һ
���жϵζ��յ�������ǣ�__________________________________________________________
����ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ ___________mL

�ɸ����������ݣ���������������Һ��Ũ�ȣ�__________mol/L
�ζ����� | ���������ml�� | ���ռ������ml�� | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 20.00 | 0.40 | 20.40 |
�ڶ��� | 20.00 | 4.00 | 24.00 |
������ | 20.00 | 2.00 | 24.10 |
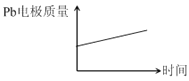
����Ŀ��Ϊ��ǿ������ʴ��,����Ǧ����Ϊ���Դ,��Al��������Pb������,���ϡ����,ʹ�����������Ĥ����Ӧԭ������:
���:Pb(s)+PbO2(s)+2H2SO4(aq)=2PbSO4(s)+2H2O(l)
����:2Al+3H2O![]() Al2O3+3H2��
Al2O3+3H2��
��������,�����ж���ȷ����( )
��� | ���� | |
A | H+����Pb�缫 | H+����Pb�缫 |
B | ÿ����3molPb | ����2molAl2O3 |
C | ����:PbO2+4H++2e-=Pb2++2H2O | ����:2Al+3H2O-6e-=Al2O3+6H+ |
D |
|
|
A. AB. BC. CD. D