��Ŀ����
2�� ȡx g NaHCO3��Na2O2�Ĺ����������һ�ܱ������м�����250�棬��ַ�Ӧ���ų��������壮����Ӧ��Ĺ���ֳ���ȫ��ͬ�����ݣ�������һ��Ͷ�뵽������BaCl2��Һ�У����ɵõ�3.94g��������һ������������ˮ��������ų�������ˮ��Һ�л����������ᣬ���������������������֮��Ĺ�ϵ��ͼ��ʾ��
ȡx g NaHCO3��Na2O2�Ĺ����������һ�ܱ������м�����250�棬��ַ�Ӧ���ų��������壮����Ӧ��Ĺ���ֳ���ȫ��ͬ�����ݣ�������һ��Ͷ�뵽������BaCl2��Һ�У����ɵõ�3.94g��������һ������������ˮ��������ų�������ˮ��Һ�л����������ᣬ���������������������֮��Ĺ�ϵ��ͼ��ʾ�����������ܷ�����һЩ��Ӧ��2Na2O2+2H2O�T4NaOH+O2����2Na2O2+2CO2�T2NaCO3+O2����NaCO3+HCl�TNaHCO3+NaCl��NaHCO3+HCl�TNaCl+CO2��+H2O��
�Իش��������⣺
��1��������������������ڱ�״���µ����Ϊ0.448 L��
��2�����ܱ��������ų�����Ļ�ѧʽ����Ӧ�����ʵ�����������ж�����������֣�
| ��ѧʽ | H2O | O2 | - |
| ���ʵ�����mol�� | 0.05 | 0.00175 | - |
��4��x=6.09��
���� Na2O2������ʵ�����NaHCO3��Ϻ����ܱ������г�ּ��ȣ����ܷ����ķ�Ӧ�У�2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O��2Na2O2+2CO2=2Na2CO3+O2��2Na2O2+2H2O=4NaOH+O2����CO2+2NaOH=Na2CO3+H2O�����ݶ��ߵ����ʵ����Ĺ�ϵ���жϲ������壻
��1������3.94g������̼�ᱵ������̼�غ����������̼�������
��2�����������غ㶨�ɼ����̼�����ơ��������Ƶ����ʵ������ٸ���̼�����Ƶķֽⷽ��ʽ��������ɶ�����̼��ˮ�ķ�Ӧ�����ݹ������Ƶ����ʵ���������������������ʵ��������ݹ���������ˮ��������̼�ķ�Ӧ����ʽ�����ʣ��ˮ�����ʵ�����
��3����ͼ��������ȷ���������ɣ�
��4����ԭ���غ�������NaHCO3��Na2O2�Ĺ��������и��ɷݵ����������������ʵ�����
��� �⣺��1����һ������������ˮ��������ų����������������ɵ�����Ϊ������̼������3.94g������̼�ᱵ������̼ԭ���غ��֪��n��CO2��=n��BaCO3��=$\frac{3.94g}{197g/mol}$=0.02mol�����Ա�״�������ɵĶ�����̼���Ϊ��V=0.02mol��22.4L/mol=0.448L��
�ʴ�Ϊ��0.448��
��2������̼�غ��֪��n��NaHCO3��=n��CO2��=0.02mol��2=0.04mol���������غ��֪n��Na2O2��=$\frac{0.2mol/L��275ml��1{0}^{-3}L/ml��2-0.04mol}{2}$=0.035mol���ɷ���ʽ��֪��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O������0.02mol�Ķ�����̼��0.02mol��ˮ���ٸ���2Na2O2+2CO2=2Na2CO3+O2��2Na2O2+2H2O=4NaOH+O2����֪��0.035mol����������ȫ��Ӧ����0.0175mol������0.02mol������̼��ȫ��Ӧ����0.02mol�������ƣ�ʣ���0.015mol����������ȫ��Ӧ����0.015molˮ�����Ի�ʣ��ˮ�����ʵ���Ϊ��0.02mol-0.015mol=0.005mol��
�ʴ�Ϊ��
| ��ѧʽ | H2O | O2 | |
| ���ʵ�����mol�� | 0.005 | 0.00175 |
��3����ͼ���֪����Ϊ175��275-175�����Թ�������ΪNaOH��Na2CO3���ʴ�Ϊ��NaOH��Na2CO3��
��4������̼�غ��֪��n��NaHCO3��=n��CO2��=0.02mol��2=0.04mol���������غ��֪n��Na2O2��=$\frac{0.2mol/L��275ml��1{0}^{-3}L/ml��2-0.04mol}{2}$=0.035mol�����������Ϊ��0.04��84+0.035��78=6.09g����x=6.09g��
�ʴ�Ϊ��6.09��
���� ���⿼���˻���ﷴӦ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ������Ӧ��ԭ��Ϊ���ؼ���ע�������غ�˼���ڻ�ѧ�����е�Ӧ�ã�����������ѧ���ķ�����������ѧ����������

| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO2����mol�� | 0.040 | 0.020 | 0.010 | 0.005 | 0.005 | 0.005 |
��2��������Ӧ�ڵ�3s��ﵽƽ�⣬��˵���÷�Ӧ�Ѵﵽƽ��״̬����bc��
a����λʱ���ڣ�����2molNO2��ͬʱ����1mol��N2O4
b��������ѹǿ���ֲ���
c��v�棨NO2��=2v����N2O4��
d���������ܶȱ��ֲ���
��3����2s�ڣ��÷�Ӧ�ų��������ա��ų�����2.7kJ������
| A�� | �ܷ���ȡ����Ӧ | B�� | �ܱ�����Cu��OH��2����Һ���� | ||
| C�� | �ܷ����Ӿ۷�Ӧ | D�� | ��ʹ��ˮ��ɫ |
| A�� | ֻ�Т� | B�� | �ں͢� | C�� | �ۺ͢� | D�� | �ں͢� |
| ������ | Na+NH4+Fe3+ |
| ������ | OH- Cl- SO42- |
| A�� | ����һ������Na+ | B�� | ����һ������NH4+ | ||
| C�� | ����һ������ Fe3+ | D�� | ����һ������SO42- |
̽��һ��̽���軯�������
��֪��������ĵ���ƽ�ⳣ�������
| ���� | HCOOH | HCN | H2CO3 |
| ����ƽ�ⳣ�� �� 25�棩 | Ki=1.77��10-4 | Ki=5.0��10-10 | Ki1=4.3��10-7 Ki2=5.6��10-11 |
��2������ѡ��������AD
A��2CN-+H2O+CO2�T2HCN+CO32-
B��2HCOOH+CO32-�T2HCOO-+H2O+CO2��
C���к͵��������pH��HCOOH��HCN����NaOH����ǰ��С�ں���
D�����������Ũ�ȵ�HCOONa��NaCN��Һ��������������ǰ��С�ں���
��3��H2O2���С���ɫ�������������ƣ�Ҳ������ˮ�е��軯���KCN���������·�Ӧʵ�֣�KCN+H2O2+H2O=A+NH3������������A�Ļ�ѧʽΪKHCO3��
��4��������CN-��ˮʱ������NaOH��Һ����pH��9ʱ����ʱc��CN-����c��HCN�������������������=����
̽�������ⶨ����ˮ���д����ٷ���
Ϊ�˲ⶨ����ˮ���д����ٷ��ʣ�ͬѧ��������ͼ��ʾװ�ý���ʵ�飮��CN-��Ũ��Ϊ0.2000mol/L�ĺ����ˮ100mL��100mL NaClO��Һ������������װ�â���ƿ�г�ַ�Ӧ����Һ©������������100mLϡH2SO4���رջ�����
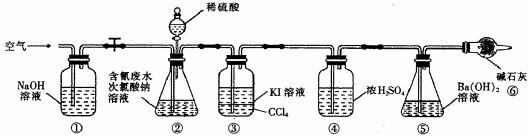
��֪װ�â��з�������Ҫ��Ӧ����Ϊ��
CN-+ClO-�TCNO-+Cl-
2CNO-+2H++3C1O-�TN2��+2CO2��+3C1-+H2O
��5���ٺ͢��������ų������ж�����̼��ʵ��ĸ��ţ�
��6����Ӧ��������ͨ�������Ŀ����ʹ���ɵ�����ȫ������װ�âݣ�
��7��Ϊ�˼����ʵ��װ�â���ƿ�к����ˮ�������İٷ��ʣ�ʵ������Ҫ�ⶨװ�âݷ�Ӧǰ�����������װ�âٵ�����ѡ����װ����ţ���
 ��������2-��-2-��ϩ
��������2-��-2-��ϩ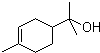 �ķ���ʽ��C10H18O
�ķ���ʽ��C10H18O ��
�� ��
��