��Ŀ����
8��������һ�ּ��߷�չDZ���������Դ����̫����Ϊ��Դ���Ȼ�ѧ���ѭ���ֽ�ˮ��һ�ָ�Ч������Ⱦ�����ⷽ�����䷴Ӧ������ͼ��ʾ��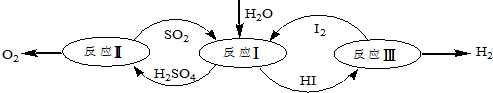
��1����ӦI�Ļ�ѧ����ʽ��SO2+2H2O+I2=H2SO4+2HI��
��2����ӦI�õ��IJ�����I2���з��룮�ò������Һ�ڹ���I2�Ĵ����»�ֳ�����-����Ũ��I2��H2SO4��ͺ���Ũ��I2��HI�㣮
�ٸ���������ʵ������˵����ȷ����ac��ѡ����ţ���
a��������Һ���ܶȴ��ڲ���
b����I2ǰ��H2SO4��Һ��HI��Һ������
c��I2��HI��Һ�б���H2SO4��Һ������
�ڱ��������Һ�ķ����ǹ۲���ɫ����ɫ���ΪHI�㣬��ɫdz��Ϊ����㣮
�۾���⣬H2SO4����c��H+����c��SO${\;}_{4}^{2-}$��=2.06��1�����ֵ����2��ԭ����������к�������I����HI����������ӣ�
���� ��1����ͼ��֪����ӦIΪ����������ⷢ��������ԭ��Ӧ���������HI��
��2���ٷֳ����㣬���ܽ��ԡ��ܶ��йأ�
���������ɫ��ͬ��
��H2SO4��c��H+����c��SO42-��=2��1����HI����������ӣ�
��� �⣺��1����ͼ��֪����ӦIΪ����������ⷢ��������ԭ��Ӧ���������HI���÷�ӦΪSO2+2H2O+I2=H2SO4+2HI���ʴ�Ϊ��SO2+2H2O+I2=H2SO4+2HI��
��2����a��������Һ���ܶȴ��ڲ�ų������²㣬��a��ȷ��
b����I2ǰ��H2SO4��Һ��HI��Һ���ܣ���ֲ��أ���b����
c��I2��HI��Һ�б���H2SO4��Һ�����ܣ�����ڲ�ͬ�ܼ����ܽ��Բ�ͬ��������ȡ����ֲ��йأ���c��ȷ��
�ʴ�Ϊ��ac��
�ڱ��������Һ�ķ����ǹ۲���ɫ����ɫ���ΪHI�㣬��ɫdz��Ϊ����㣬�ʴ�Ϊ���۲���ɫ����ɫ���ΪHI�㣬��ɫdz��Ϊ����㣻
��H2SO4����c��H+����c��SO42-��=2.06��1�����ֵ����2��ԭ����������к�������HI����HI����������ӣ��ʴ�Ϊ��������к�������I����HI����������ӣ�
���� ���⿼����������ᴿ����ѧƽ��ȣ�Ϊ��Ƶ���㣬���շ����ķ�Ӧ��ƽ��Ӱ������Ϊ���Ĺؼ������ط�����Ӧ���������ۺϿ��飬��Ŀ�Ѷ��еȣ�

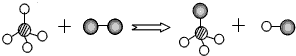
| A�� | �÷�Ӧ�й�������Ԫ�� | B�� | ͼ�з�Ӧ�ﶼ�ǻ����� | ||
| C�� | �÷�Ӧ�ı�����ԭ�ӵ�������� | D�� | �÷�Ӧǰ����ӵ�������ı� |
| A�� | ţ�̡���ɫ���������ڽ��� | |
| B�� | Ư�ۡ�ʯӢ�����ڴ����� | |
| C�� | �Ȼ�李������ᶼ����ǿ����� | |
| D�� | �����ǡ������ʶ����ڸ߷��ӻ����� |
| A�� | ú�ĸ��� | B�� | ʯ�͵ij�ѹ���� | ||
| C�� | ʯ�͵Ĵ��ѻ� | D�� | �Ի�����Ϊԭ������ |
 ��֪����CH2CH2Br+NaCN$\stackrel{��}{��}$CH3CH2CN+NaBr��
��֪����CH2CH2Br+NaCN$\stackrel{��}{��}$CH3CH2CN+NaBr��