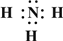��Ŀ����
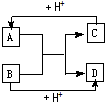 ��֪A��B��C��D�ֱ�����ѧ��ѧ�г��������ֲ�ͬ���ӣ�����֮��������ͼ��ʾ��Ӧ��ϵ��
��֪A��B��C��D�ֱ�����ѧ��ѧ�г��������ֲ�ͬ���ӣ�����֮��������ͼ��ʾ��Ӧ��ϵ����1�����A��B��C��D����10�������ӣ�
����д��A�Ļ�ѧʽ
NH4+ ����HF��
NH4+ ����HF��
��A��B��Ӧ����C��D�����ӷ�Ӧ����ʽ
NH4++OH-�TNH3��+H2O ����HF+OH-�TF-+H2O ��
NH4++OH-�TNH3��+H2O ����HF+OH-�TF-+H2O ��
��2�����A��C����18���ӵ����ӣ�B��D ����10�������ӣ���д��C�ĵ���ʽ
 ��
��
 ��
��
��������10��������Na+��Mg2+��Al3+��NH4+��H3O+��N3-��O2-��F-��OH-��NH2-��Ne��HF��H2O��NH3��CH4�����ҳ�����ͼʾ�����ӣ�AΪNH4+��BΪOH-��CΪNH3��DΪH2O��18����������K+��Ca2+��P3-��S2-��HS-��Cl-��Ar��HCl��H2S��PH3��SiH4��F2��H2O2��C2H6��CH3OH��CH3F��N2H4�ȣ���ͼʾ��A��C��18�������ӣ�B��D��10�������ӣ���AΪH2S��BΪOH-��CΪHS-��S2-��DΪH2O��
����⣺��1�����A��B��C��D����10�������ӣ����10�������Ľṹ���������ж�Ϊ��AΪNH4+��BΪOH-��CΪNH3��DΪH2O����ӦΪ��NH4++OH-=NH3+H2O����Ϊ��HF+OH-�TF-+H2O
��A�Ļ�ѧʽΪ��NH4+ ����HF��
��A��B��Ӧ����C��D�����ӷ�Ӧ����ʽΪ��NH4++OH-�TNH3��+H2O ����HF+OH-�TF-+H2O ��
�ʴ�Ϊ��NH4+ ����HF����NH4++OH-�TNH3��+H2O ����HF+OH-�TF-+H2O ��
��2�����A��C����18���ӵ����ӣ�B��D ����10�������ӣ�����������������ƶϣ�AΪH2S��BΪOH-��CΪHS-��S2-��DΪH2O������C�ĵ���ʽΪ�� ����
���� ��
��
�ʴ�Ϊ�� ����
����
��A�Ļ�ѧʽΪ��NH4+ ����HF��
��A��B��Ӧ����C��D�����ӷ�Ӧ����ʽΪ��NH4++OH-�TNH3��+H2O ����HF+OH-�TF-+H2O ��
�ʴ�Ϊ��NH4+ ����HF����NH4++OH-�TNH3��+H2O ����HF+OH-�TF-+H2O ��
��2�����A��C����18���ӵ����ӣ�B��D ����10�������ӣ�����������������ƶϣ�AΪH2S��BΪOH-��CΪHS-��S2-��DΪH2O������C�ĵ���ʽΪ��
 ����
���� ��
���ʴ�Ϊ��
 ����
����
���������⿼���˳���10��������18�������Ľṹ������Ӧ�ã���Ҫ�������ӷ�Ӧ�Ľṹ��������Ϥ10��������18������ �ǽ���ؼ���

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
��֪A��B��C��D�ֱ���Cu��Ag��Fe��Al���ֽ����е�һ�֣���֪��A��C������ϡ���ᷴӦ�ų����壻��B��D�������η�Ӧ���û�������D����C��ǿ�Ӧ�ų����壬�ɴ˿����ƶ�A��B��C��D�����ǣ�������
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |
 ��2013?����ģ�⣩��һ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������X����������Y���ڲ��������ȣ�Yԭ�ӵ������������Ǵ�����������������Z��L�ǿ����к������Ķ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ��ش��������⣺
��2013?����ģ�⣩��һ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������X����������Y���ڲ��������ȣ�Yԭ�ӵ������������Ǵ�����������������Z��L�ǿ����к������Ķ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ��ش��������⣺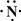 ��
��