��Ŀ����
 ��2013?����ģ�⣩��һ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������X����������Y���ڲ��������ȣ�Yԭ�ӵ������������Ǵ�����������������Z��L�ǿ����к������Ķ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ��ش��������⣺
��2013?����ģ�⣩��һ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������X����������Y���ڲ��������ȣ�Yԭ�ӵ������������Ǵ�����������������Z��L�ǿ����к������Ķ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ��ش��������⣺��1��L��Ԫ������Ϊ
��
��
������Ԫ�ص�ԭ�Ӱ뾶��С�����˳���ǣ���Ԫ�ط��ű�ʾ��H��O��N��C��Al
H��O��N��C��Al
����2��Z��X��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���A��B���õ���ʽ��ʾA���γɹ���
3H?+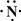 ��
��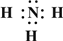
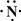 ��
��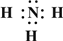
3H?+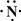 ��
��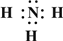
��B�Ľṹʽ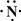 ��
��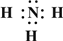


��3������Se��������������Ԫ�أ���Lͬһ���壬Seԭ�ӱ�Lԭ�Ӷ��������Ӳ㣬��Se��ԭ������Ϊ
34
34
��������������Ӧ��ˮ���ﻯѧʽΪH2SeO4
H2SeO4
������2��5����Ԫ�ص��ʷֱ���H2��Ӧ����1mol��̬�⻯��ķ�Ӧ�����£���ʾ����1mol�����ⷴӦ�ȵ���b
b
������ĸ���ţ���a��+99.7kJ?mol-1 b��+29.7kJ?mol-1c��-20.6kJ?mol-1 d��-241.8kJ?mol-1��4��һ�������£�M��TiO2��C��ʯī����Ӧֻ�����Һ�̼���ѣ�TiC�������߾�ΪijЩ���½ṹ�մɵ���Ҫ�ɷ֣���֪���÷�Ӧ����lmol��ʱ�ų�536kJ���������Ȼ�ѧ����ʽΪ
4Al��s��+3TiO2��s��+3C��s��ʯī��=2Al2O3��s��+3TiC��s����M=-1072g/mol
4Al��s��+3TiO2��s��+3C��s��ʯī��=2Al2O3��s��+3TiC��s����M=-1072g/mol
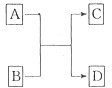
����������֪A��B��C��D�ֱ����ɶ�����Ԫ��ԭ����ɵ�������������֮������ͼ��ʾ��ת���ϵ����A���ֺ���18���ӵ�����C��һ�ֺ���10���ӵ�������������и��⣺
��1����A��D�ֱ���������̬���ʷ��ӣ�д��A��B��Ӧ�Ļ�ѧ����ʽ
2F2+2H2O=4HF+O2
2F2+2H2O=4HF+O2
����2����B��D��ͬ����ĵ��ʷ��ӣ�д��C�Ļ�ѧʽ
H2O
H2O
����3����B��һ�ֺ��ĺ�l8���ӵķ��ӣ����D��һ����̬���ʷ��ӣ�B�Ľṹ��ʽΪ
H-O-O-H
H-O-O-H
����4����A��B���Ǻ�2��ԭ�Ӻ˵���������B�к���10�����ӣ�D�к���18�����ӣ���A��B֮�䷢�������ӷ�Ӧ����ʽΪ
HS-+OH-=S2-+H2O
HS-+OH-=S2-+H2O
����5����D��һ�ֺ���22���ӵķ��ӣ��������ͼ��ϵ��A��������
CH3OH��CH3-CH3
CH3OH��CH3-CH3
��д�����ʵĻ�ѧʽ��������л�����д��Ӧ�Ľṹ��ʽ������������һ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������Z��L�ǿ����к������Ķ���Ԫ�أ���ZΪNԪ�أ�LΪOԪ�أ�
M�ǵؿ��к�����ߵĽ���Ԫ�أ���MΪAlԪ�أ�Yԭ�ӵ������������Ǵ�����������������Y��2�����Ӳ㣬����������Ϊ4����YΪCԪ�أ�X����������Y���ڲ��������ȣ�X����������2�����ӣ���XΪHԪ�أ�
���������ƶ�10��������˼·�����з�����������18��һ��֣���18���9+9���ҳ�F2���ʹ18���ӵ�����һ���ռ䡱��9���ӵĿ�����F��OH��NH2��CH3�ȣ�����Щ9�������ٽ�������ɵã����ӣ�F2��HO-OH��NH2-NH2��CH3-CH3��CH3-F��CH3-OH�������ӣ�N2H5+��N2H62+�������ӣ�O22-���ٽ�ϳ�����10���ӡ�18������=����֮��ķ�Ӧ�жϣ�
��1��A��D�ֱ���������̬���ʷ��ӣ���ת���ϵ��֪��AΪF2��BΪH2O��CΪHF��DΪO2���������⣮
��2����B��D��ͬ����ĵ��ʷ��ӣ�AΪH2S��BΪO2��CΪˮH2O��DΪS���������⣮
��3����B��һ�ֺ��ĺ�l8���ӵķ��ӣ����D��һ����̬���ʷ��ӣ���ת���ϵ��֪��AΪN2H4��BΪH2O2�����߷�Ӧ����D��N2����C��H2O���������⣮
��4����A��B���Ǻ�2��ԭ�Ӻ˵���������B�к���10�����ӣ�D�к���18�����ӣ���AΪHS-��BΪOH-��CΪˮH2O��DΪS2-�������⣮
��5����D��һ�ֺ���22���ӵķ��ӣ�AΪ18����������������Ӧ������CO2��H2O��CO2����22�����ӣ��������⣬��DΪCO2��CΪH2O��AΪ18�����л�����ԣ�
M�ǵؿ��к�����ߵĽ���Ԫ�أ���MΪAlԪ�أ�Yԭ�ӵ������������Ǵ�����������������Y��2�����Ӳ㣬����������Ϊ4����YΪCԪ�أ�X����������Y���ڲ��������ȣ�X����������2�����ӣ���XΪHԪ�أ�
���������ƶ�10��������˼·�����з�����������18��һ��֣���18���9+9���ҳ�F2���ʹ18���ӵ�����һ���ռ䡱��9���ӵĿ�����F��OH��NH2��CH3�ȣ�����Щ9�������ٽ�������ɵã����ӣ�F2��HO-OH��NH2-NH2��CH3-CH3��CH3-F��CH3-OH�������ӣ�N2H5+��N2H62+�������ӣ�O22-���ٽ�ϳ�����10���ӡ�18������=����֮��ķ�Ӧ�жϣ�
��1��A��D�ֱ���������̬���ʷ��ӣ���ת���ϵ��֪��AΪF2��BΪH2O��CΪHF��DΪO2���������⣮
��2����B��D��ͬ����ĵ��ʷ��ӣ�AΪH2S��BΪO2��CΪˮH2O��DΪS���������⣮
��3����B��һ�ֺ��ĺ�l8���ӵķ��ӣ����D��һ����̬���ʷ��ӣ���ת���ϵ��֪��AΪN2H4��BΪH2O2�����߷�Ӧ����D��N2����C��H2O���������⣮
��4����A��B���Ǻ�2��ԭ�Ӻ˵���������B�к���10�����ӣ�D�к���18�����ӣ���AΪHS-��BΪOH-��CΪˮH2O��DΪS2-�������⣮
��5����D��һ�ֺ���22���ӵķ��ӣ�AΪ18����������������Ӧ������CO2��H2O��CO2����22�����ӣ��������⣬��DΪCO2��CΪH2O��AΪ18�����л�����ԣ�
����⣺��һ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������Z��L�ǿ����к������Ķ���Ԫ�أ���ZΪNԪ�أ�LΪOԪ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ���MΪAlԪ�أ�Yԭ�ӵ������������Ǵ�����������������Y��2�����Ӳ㣬����������Ϊ4����YΪCԪ�أ�X����������Y���ڲ��������ȣ�X����������2�����ӣ���XΪHԪ�أ�
��1��������������֪��LΪ��Ԫ�أ�ͬ������ԭ����������ԭ�Ӱ뾶��С�����Ӳ�Խ��ԭ�Ӱ뾶Խ����������Ԫ�ص�ԭ�Ӱ뾶��С�����˳����H��O��N��C��Al��
�ʴ�Ϊ������H��O��N��C��Al��
��2��ZΪNԪ�ء�XΪHԪ�أ�Z��X��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���AΪNH3��BΪN2H4���õ���ʽ��ʾNH3���γɹ���Ϊ3H?+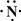 ��
��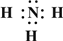 ��N2H4�ĽṹʽΪ
��N2H4�ĽṹʽΪ ���ʴ�Ϊ��3H?+
���ʴ�Ϊ��3H?+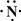 ��
��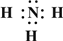 ��
�� ��
��
��3������Se����OԪ��ͬһ���壬����������Ϊ6��Seԭ�ӱ�Lԭ�Ӷ��������Ӳ㣬��4�����Ӳ㣬���������Ϊ2��8��18��6����Se��ԭ������Ϊ2+8+18+6=34������ϼ�Ϊ+6�ۣ�����������Ӧ��ˮ���ﻯѧʽΪH2SeO4��
���϶��·ǽ����������뷴Ӧ�ľ��ҳ̶ȼ�������ӦԽ��Խ���ѣ���Ӧ��Խ��Խ������1mol�����ⷴӦ�ȵ�Ӧ��+29.7kJ?mol-1��
�ʴ�Ϊ��34��H2SeO4��b��
��4��һ�������£�Al��TiO2��C��ʯī����Ӧֻ�����Һ�̼���ѣ�TiC�������߾�ΪijЩ���½ṹ�մɵ���Ҫ�ɷ֣���ΪAl2O3���÷�Ӧ����lmolAl2O3ʱ�ų�536kJ���������Ȼ�ѧ����ʽΪ 4Al��s��+3TiO2��s��+3C��s��ʯī��=2Al2O3��s��+3TiC��s����M=-1072g/mol��
�ʴ�Ϊ��4Al��s��+3TiO2��s��+3C��s��ʯī��=2Al2O3��s��+3TiC��s����M=-1072g/mol��
��������1��AΪF2��BΪH2O��CΪHF��DΪO2����Ӧ����ʽΪ2F2+2H2O=4HF+O2���ʴ�Ϊ��2F2+2H2O=4HF+O2��
��2����B��D��ͬ����ĵ��ʷ��ӣ�AΪH2S��BΪO2��CΪˮH2O��DΪS���������⣬�ʴ�Ϊ��H2O��
��3����B��һ�ֺ��ĺ�l8���ӵķ��ӣ����D��һ����̬���ʷ��ӣ���ת���ϵ��֪��AΪN2H4��BΪH2O2�����߷�Ӧ����D��N2����C��H2O���������⣬H2O2�ĽṹʽΪH-O-O-H��
�ʴ�Ϊ��H-O-O-H��
��4����A��B���Ǻ�2��ԭ�Ӻ˵���������B�к���10�����ӣ�D�к���18�����ӣ���AΪHS-��BΪOH-��CΪˮH2O��DΪS2-�������⣬��Ӧ���ӷ���ʽΪHS-+OH-=S2-+H2O��
�ʴ�Ϊ��HS-+OH-=S2-+H2O��
��5����D��һ�ֺ���22���ӵķ��ӣ�AΪ18����������������Ӧ������CO2��H2O��CO2����22�����ӣ�AΪCH3OH��CH3-CH3���ʴ�Ϊ��CH3OH��CH3-CH3��
��1��������������֪��LΪ��Ԫ�أ�ͬ������ԭ����������ԭ�Ӱ뾶��С�����Ӳ�Խ��ԭ�Ӱ뾶Խ����������Ԫ�ص�ԭ�Ӱ뾶��С�����˳����H��O��N��C��Al��
�ʴ�Ϊ������H��O��N��C��Al��
��2��ZΪNԪ�ء�XΪHԪ�أ�Z��X��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���AΪNH3��BΪN2H4���õ���ʽ��ʾNH3���γɹ���Ϊ3H?+
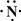 ��
��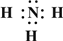 ��N2H4�ĽṹʽΪ
��N2H4�ĽṹʽΪ ���ʴ�Ϊ��3H?+
���ʴ�Ϊ��3H?+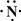 ��
��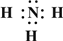 ��
�� ��
����3������Se����OԪ��ͬһ���壬����������Ϊ6��Seԭ�ӱ�Lԭ�Ӷ��������Ӳ㣬��4�����Ӳ㣬���������Ϊ2��8��18��6����Se��ԭ������Ϊ2+8+18+6=34������ϼ�Ϊ+6�ۣ�����������Ӧ��ˮ���ﻯѧʽΪH2SeO4��
���϶��·ǽ����������뷴Ӧ�ľ��ҳ̶ȼ�������ӦԽ��Խ���ѣ���Ӧ��Խ��Խ������1mol�����ⷴӦ�ȵ�Ӧ��+29.7kJ?mol-1��
�ʴ�Ϊ��34��H2SeO4��b��
��4��һ�������£�Al��TiO2��C��ʯī����Ӧֻ�����Һ�̼���ѣ�TiC�������߾�ΪijЩ���½ṹ�մɵ���Ҫ�ɷ֣���ΪAl2O3���÷�Ӧ����lmolAl2O3ʱ�ų�536kJ���������Ȼ�ѧ����ʽΪ 4Al��s��+3TiO2��s��+3C��s��ʯī��=2Al2O3��s��+3TiC��s����M=-1072g/mol��
�ʴ�Ϊ��4Al��s��+3TiO2��s��+3C��s��ʯī��=2Al2O3��s��+3TiC��s����M=-1072g/mol��
��������1��AΪF2��BΪH2O��CΪHF��DΪO2����Ӧ����ʽΪ2F2+2H2O=4HF+O2���ʴ�Ϊ��2F2+2H2O=4HF+O2��
��2����B��D��ͬ����ĵ��ʷ��ӣ�AΪH2S��BΪO2��CΪˮH2O��DΪS���������⣬�ʴ�Ϊ��H2O��
��3����B��һ�ֺ��ĺ�l8���ӵķ��ӣ����D��һ����̬���ʷ��ӣ���ת���ϵ��֪��AΪN2H4��BΪH2O2�����߷�Ӧ����D��N2����C��H2O���������⣬H2O2�ĽṹʽΪH-O-O-H��
�ʴ�Ϊ��H-O-O-H��
��4����A��B���Ǻ�2��ԭ�Ӻ˵���������B�к���10�����ӣ�D�к���18�����ӣ���AΪHS-��BΪOH-��CΪˮH2O��DΪS2-�������⣬��Ӧ���ӷ���ʽΪHS-+OH-=S2-+H2O��
�ʴ�Ϊ��HS-+OH-=S2-+H2O��
��5����D��һ�ֺ���22���ӵķ��ӣ�AΪ18����������������Ӧ������CO2��H2O��CO2����22�����ӣ�AΪCH3OH��CH3-CH3���ʴ�Ϊ��CH3OH��CH3-CH3��
���������⿼��Ԫ�ػ������ƶϡ�����10���ӡ�18�����������ʣ�Ԫ�������ɣ����û�ѧ����ȣ��ѶȺܴ��ƶ�Ԫ���������ǹؼ���ѧ��Ҫ��������10���ӡ�18����������֮��ķ�Ӧ��

��ϰ��ϵ�д�
�����Ŀ
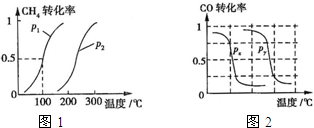 ��2013?����ģ�⣩�״���һ�ֺܺõ�ȼ�ϣ���ҵ����CH4��H2OΪԭ�ϣ�ͨ��������Ӧ��͢����Ʊ��״���
��2013?����ģ�⣩�״���һ�ֺܺõ�ȼ�ϣ���ҵ����CH4��H2OΪԭ�ϣ�ͨ��������Ӧ��͢����Ʊ��״���


