��Ŀ����
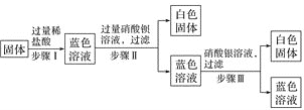
����Ŀ��ijͬѧ������ͼ��ʾ������ȡ�����еĵ�Ԫ�ء�
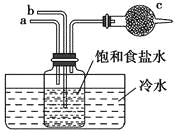
��1������������պ������õ�������___������ţ���
a.�Թ� b.���� c.�ձ� d.��ƿ
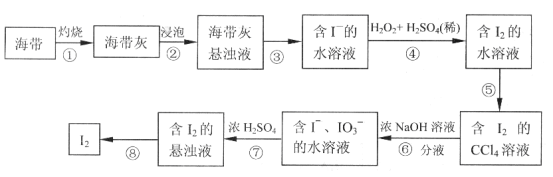
��2����������������ữ��H2O2��Һ��I-������I2����Ӧ�����ӷ���ʽ��__��
��3��������в��õķ��뷽����___��
��4��������ݵõ���I2��CCl4��Һ������ȡ����ޢߵ�Ŀ����__��
��5������ߵķ�Ӧ�У���1molIO3-������Ӧʱ��ת�Ƶ��ӵ����ʵ�����__mol��
���𰸡�b 2I-+H2O2+2H+=I2+2H2O ��ȡ����Һ ��I2��CCl4��Һ�з����I2 5
��������
(1)����������պ���ʱ�������������������£��Ӷ�ȷ�����õ�������
(2)������У��������ữ��H2O2��Һ��I-������I2��ͬʱ���������κ�ˮ��
(3)������У��ӵ�ˮ����ȡ�⣬Ӧ������ȡ����
(4)������ݵõ���I2��CCl4��Һ�õ����ǵ�����Ȼ�̼��Һ�����轫����������
(5)����ߵķ�Ӧ�У�IO3-������Ӧʱ����+5�۽���Ϊ0�ۡ�
(1)����������պ���ʱ�������������������£��Ӷ�ȷ�����õ�����Ϊ��������Ϊ��b��
(2)������У��������ữ��H2O2��Һ��I-������I2��ͬʱ���������κ�ˮ����Ӧ�����ӷ���ʽΪ2I-+H2O2+2H+=I2+2H2O����Ϊ��2I-+H2O2+2H+=I2+2H2O��
(3)������У��ӵ�ˮ����ȡ�⣬Ӧ������ȡ����Ȼ���Һ����Ϊ����ȡ����Һ��
(4)������ݵõ���I2��CCl4��Һ�õ����ǵ�����Ȼ�̼��Һ�����轫�������������Լ�����ȡ����ޢߵ�Ŀ���Ǵ�I2��CCl4��Һ�з����I2����Ϊ����I2��CCl4��Һ�з����I2��
(5)����ߵķ�Ӧ�У�IO3-������Ӧʱ����+5�۽���Ϊ0�ۣ����ϼ۽���5�ۣ��ʵ�1molIO3-������Ӧʱ��ת�Ƶ��ӵ����ʵ�����5mol����Ϊ��5��

����Ŀ�����ݻ�Ϊ2 L���ܱ������м�����������̿��NO��������Ӧ��C(s)+2NO(g)![]() N2(g)+CO2(g)����H<0��NO��N2�����ʵ����仯���±���ʾ��
N2(g)+CO2(g)����H<0��NO��N2�����ʵ����仯���±���ʾ��
���ʵ���/mol | T1/�� | T2/�� | |||||
0 | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min | |
NO | 2.0 | 1.16 | 0.80 | 0.80 | 0.50 | 0.40 | 0.40 |
N2 | 0 | 0.42 | 0.60 | 0.60 | 0.75 | 0.80 | 0.80 |
��ش��������⣺
(1) 0��5 min�ڣ���CO2��ʾ�ĸ÷�Ӧ����v(CO2)=____���������µ�ƽ�ⳣ��K��____��
(2) ��15 min���¶ȵ�����T2�����ݱ仯���ϱ���ʾ����T1___T2(��������������������=�� )��
(3)��30 minʱ������T2���䣬����������ټ�������ַ�Ӧ������2 mol����ÿ��淴Ӧ���մ�ƽ��ʱNO��ת������=_______��