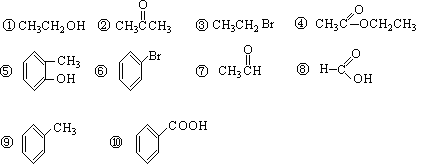
��Ŀ����
AΪij�л��ϳɵ��м��壬��������֧������һ�������·�����ȥ��Ӧ���ܵõ����ֻ�Ϊͬ���칹��IJ��B�����е�һ�֣���������ȡ�ϳ���֬��Ⱦ�ϵȶ��ֻ�����Ʒ��A�ܷ�������ͼ��ʾ�ı仯��
��֪ (ע��R��R´Ϊ����)
(ע��R��R´Ϊ����)
�Իش�
��1����A��ͬ���칹����
a��д��һ�־������ԵĽṹ��ʽ____________________��
b���ܷ���ˮ�ⷴӦ����____________�֡�
��2�� C��D�ķ�Ӧ������___________��E��F�ķ�Ӧ������___________��
a��������Ӧ b����ԭ��Ӧ c���ӳɷ�Ӧ d��ȡ����Ӧ
��3�� D�Ľṹ��ʽ��_____________��
��4��д��E���ɸ߾���Ļ�ѧ����ʽ��______________��
��5��������C��ȥ������ˮ�γɺ��а�Ԫ����M��д��M�Ľṹ��ʽ��________________________��
��1��a��CH3CH2CH2COOH�ۻ�(CH3)2CHCOOH�� ��2�֣� b��4 ��3�֣�
��2�� a �� b��c ����2�֣�
��3�� CH3COCH2COOH ��2�֣�
��4�� nCH3CH��CHCH2OH
 ��3�֣�
��3�֣�
��5��  ��3�֣�
��3�֣�
�������������A�������Ҳ���ˮ�⣬��A�в����������Ȼ���A�ܷ���������Ӧ����A����ȩ����A�ܷ�����ȥ��Ӧ����A�к��д��ǻ���A������ȥ��Ӧ����B��B�к���һ������̼̼˫������B�Ľṹ��ʽΪ��CH3CH=CHCHO��A��������֧������һ�������·�����ȥ��Ӧ�����ܵõ����ֻ�Ϊͬ���칹��IJ����A�Ľṹ��ʽΪ��CH3CH��OH��CH2CHO��A��������Һ��������C��C�Ľṹ��ʽΪ��CH3CH��OH��CH2COOH��C�ں��ʵ���������Ӧ����D�����������Ϣ֪��D�Ľṹ��ʽΪ��CH3COCH2COOH��B�����������ӳɷ�Ӧ����F��F�Ľṹ��ʽΪ��CH3CH2CH2CH2OH��B����ԭ����E��E��ʹ��ˮ��ɫ��˵������̼̼˫����������������Ӧ��˵������ȩ������E�Ľṹ��ʽΪ��CH3CH=CHCH2OH����1��������������֪AΪCH3CH��OH��CH2CHO��A��ͬ���칹�壺a���������ԣ�����-COOH����ΪCH3CH2CH2COOH[��CH3��2CHCOOH]����b���ܷ���ˮ�ⷴӦ��������������ΪCH3CH2COOCH3[��CH3COOCH2CH3��HCOOCH2CH2CH3��HCOOCH��CH3��2]���ʴ�Ϊ��CH3CH2CH2COOH[��CH3��2CHCOOH]��CH3CH2COOCH3[��CH3COOCH2CH3��HCOOCH2CH2CH3��HCOOCH��CH3��2]����2��C��D��CH3CH��OH��CH2COOH����������Ӧ����CH3COCH2CHO����ѡa��E��F��CH3CH=CHCH2OH�����������ӳɷ�Ӧ����CH3CH2CH2CH2OH�����ڻ�ԭ��Ӧ����ѡbc���ʴ�Ϊ��a��bc����3��������������֪��D�Ľṹ��ʽ��CH3COCH2COOH���ʴ�Ϊ��CH3COCH2COOH����4��E���ɸ߾���Ļ�ѧ����ʽΪ��nCH3CH=CHCH2OH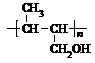 ���ʴ�Ϊ��nCH3CH=CHCH2OH��
���ʴ�Ϊ��nCH3CH=CHCH2OH��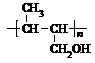 ����5��������CH3CH��OH��CH2COOH��ȥ������ˮ�γɺ��а�Ԫ����M����M�Ľṹ��ʽΪ��
����5��������CH3CH��OH��CH2COOH��ȥ������ˮ�γɺ��а�Ԫ����M����M�Ľṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���㣺���⿼���л�����ƶϣ���A�ķ���ʽ�뷴Ӧ�ص�ȷ��B�Ľṹ���ƶϵ�ͻ�ƿڣ����չ����ŵ�������ת���ǹؼ�����ѧ�����������нϸߵ�Ҫ���Ѷ��еȡ�

 �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�

 �� �����
�� �����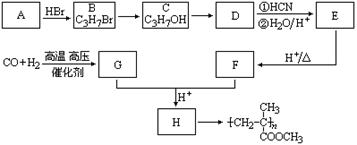

 ��5��F��G�ķ�Ӧ������ ��
��5��F��G�ķ�Ӧ������ ��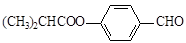 G��һ�ֺϳ�·�����£�
G��һ�ֺϳ�·�����£�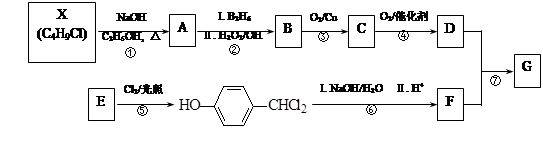
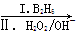 RCH2CH2OH��ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ�����ش��������⣺
RCH2CH2OH��ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ�����ش��������⣺
 N��ԭΪ-CH2NH2
N��ԭΪ-CH2NH2
 ���л���F�Ƕ�����ӵ�һ��ͬ���칹�壬���л�����ԭ
���л���F�Ƕ�����ӵ�һ��ͬ���칹�壬���л�����ԭ