��Ŀ����
| ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ��������¶ȶԷ�Ӧ���ʵ�Ӱ�죮�������·������ʵ�飮����˵������ȷ���� ��������
|
������A����ȡ���Ʊ�����������Ũ��Խ���¶�Խ�ߡ�ʹ�ô�����Ӧ����Խ�죻
B����ȡ���Ʊ�����������Ũ��Խ���¶�Խ�ߡ�ʹ�ô�����Ӧ����Խ�죻
C���ɱ������ݿ�֪��ʵ��ڡ��ܵIJ�֮ͬ���Ǣ���ʹ�ô������¶ȱ�ʵ����иߣ�
D����������רһ�ԣ�FeCl3���Դ�H2O2�ֽ⣬����һ����������Ӧ�Ĵ�����FeCl3��Zn�뷢����Ӧ�����ܴ�Zn�����ᷴӦ��
B����ȡ���Ʊ�����������Ũ��Խ���¶�Խ�ߡ�ʹ�ô�����Ӧ����Խ�죻
C���ɱ������ݿ�֪��ʵ��ڡ��ܵIJ�֮ͬ���Ǣ���ʹ�ô������¶ȱ�ʵ����иߣ�
D����������רһ�ԣ�FeCl3���Դ�H2O2�ֽ⣬����һ����������Ӧ�Ĵ�����FeCl3��Zn�뷢����Ӧ�����ܴ�Zn�����ᷴӦ��
����⣺A���ɱ������ݿ�֪��ʵ�����Ũ����ߡ��¶������ʹ�ô������ʷ�Ӧ������죬��A��ȷ��
B���ɱ������ݿ�֪��ʵ�����Ũ����͡��¶������û��ʹ�ô������ʷ�Ӧ������������B��ȷ��
C���ɱ������ݿ�֪��ʵ��ڡ��ܵIJ�֮ͬ���Ǣ���ʹ�ô������¶ȱ�ʵ����иߣ���ʵ�������С��ʵ��ܵ�ԭ���д������¶ȵ�Ӱ�죬��C��ȷ��
D����������רһ�ԣ�FeCl3���Դ�H2O2�ֽ⣬����һ����������Ӧ�Ĵ�����FeCl3��Zn�뷢����Ӧ�����ܴ�Zn�����ᷴӦ����D����
��ѡD��
B���ɱ������ݿ�֪��ʵ�����Ũ����͡��¶������û��ʹ�ô������ʷ�Ӧ������������B��ȷ��
C���ɱ������ݿ�֪��ʵ��ڡ��ܵIJ�֮ͬ���Ǣ���ʹ�ô������¶ȱ�ʵ����иߣ���ʵ�������С��ʵ��ܵ�ԭ���д������¶ȵ�Ӱ�죬��C��ȷ��
D����������רһ�ԣ�FeCl3���Դ�H2O2�ֽ⣬����һ����������Ӧ�Ĵ�����FeCl3��Zn�뷢����Ӧ�����ܴ�Zn�����ᷴӦ����D����
��ѡD��
���������⿼��Ӱ�컯ѧ��Ӧ���ʵ����ء����ݷ��������ȣ��Ѷ��еȣ�ע����Ʊ����������ã�

��ϰ��ϵ�д�
�����Ŀ
H2O2��ʵ���ҳ��õ�һ�ֻ�ѧ�Լ���ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�죮�ڳ����°������·������ʵ�飮
��1��ʵ��ٺ͢ڵ�Ŀ���� ��
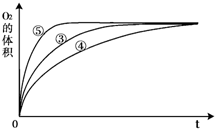
��2��ʵ��ۡ��ܡ����У�������������������ʱ��仯�Ĺ�ϵ��ͼ1��������ͼ�ܹ��ó��Ľ����� ��
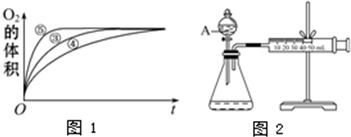
��3��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�������ͼ2��ʾ��ʵ��װ�ý���ʵ�飮
ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ���ʵ������Ҫ���������� ��
��4��H2O2������H2SO4 �ữ��FeSO4�����ӷ���ʽΪ ��
| ʵ���� | ��Ӧ�� | ���� |
| �� | 10mL2% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | 1mL0.1mol?L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����HCl��Һ | 1mL0.1mol?L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����NaOH��Һ | 1mL0.1mol?L-1FeCl3��Һ |
��2��ʵ��ۡ��ܡ����У�������������������ʱ��仯�Ĺ�ϵ��ͼ1��������ͼ�ܹ��ó��Ľ�����
��3��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�������ͼ2��ʾ��ʵ��װ�ý���ʵ�飮
ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ���ʵ������Ҫ����������
��4��H2O2������H2SO4 �ữ��FeSO4�����ӷ���ʽΪ

ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ������Է�Ӧ���ʵ�Ӱ�죮�ڳ����°������·������ʵ�飮ʵ��ڵķ�Ӧ��Ӧ��������
| ʵ���� | ��Ӧ�� | ���� |
| �� | 10mL 2% H2O2��Һ | �� |
| �� | �� | |
| �� | 10mL 5% H2O2��Һ | MnO2 ���� |
| A��5mL 2% H2O2��Һ |
| B��10mL 5% H2O2��Һ |
| C��10mL 2% H2O2��Һ |
| D��5mL 10% H2O2��Һ |
H2O2��ʵ���ҳ��õ�һ�ֻ�ѧ�Լ���
I��ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�죮�ڳ����°������·������ʵ�飮
��1��ʵ��ٺ͢ڵ�Ŀ���� ��ʵ��ʱ����û�й۲쵽������������ó����ۣ�������ʾ��ͨ��������H2O2�ȶ������ֽ⣮Ϊ�˴ﵽʵ��Ŀ�ģ����ԭʵ�鷽���ĸĽ��� ��
��2��ʵ��ۡ��ܡ����У�������������������ʱ��仯�Ĺ�ϵ��ͼ1������ͼ��1���ó���ʵ������� ��

��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�������ͼ2��ʾ��ʵ��װ�ý���ʵ�飮
��1��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ���ʵ������Ҫ������������ ��
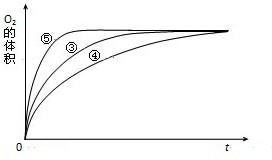
��2������0.1g mol MnO2��ĩ��50mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ3��ʾ�����ͷ�Ӧ���ʱ仯��ԭ�� ������H2O2�ij�ʼ���ʵ���Ũ�� ����������λ��Ч���֣�
I��ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�죮�ڳ����°������·������ʵ�飮
| ʵ���� | ��Ӧ�� | ���� |
| �� | 10mL2% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | �� |
| �� | 10mL5% H2O2��Һ | 1mL0.1mol?L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����HCl��Һ | 1mL0.1mol?L-1FeCl3��Һ |
| �� | 10mL5% H2O2��Һ+����NaOH��Һ | 1mL0.1mol?L-1FeCl3��Һ |
��2��ʵ��ۡ��ܡ����У�������������������ʱ��仯�Ĺ�ϵ��ͼ1������ͼ��1���ó���ʵ�������

��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�������ͼ2��ʾ��ʵ��װ�ý���ʵ�飮
��1��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ���ʵ������Ҫ������������
��2������0.1g mol MnO2��ĩ��50mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ3��ʾ�����ͷ�Ӧ���ʱ仯��ԭ��
 ��2011?˳����ģ�⣩ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�죮�ڳ����°������·������ʵ�飮
��2011?˳����ģ�⣩ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�죮�ڳ����°������·������ʵ�飮 O2��+2H2O
O2��+2H2O