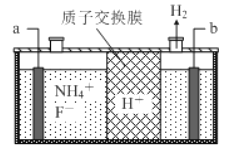
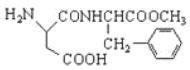
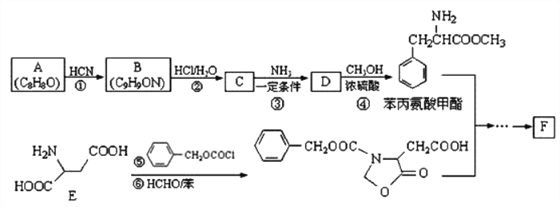
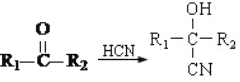
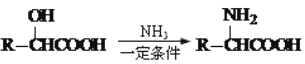
��Ŀ����
����Ŀ���л��� A������ʳƷ��ҵ����֪ 9.0 g A ������ O2�г��ȼ�գ������ɵĻ����������ͨ��������Ũ����ͼ�ʯ�ң��ֱ����� 5.4g �� 13.2 g��������ʣ������Ϊ O2��
(1) A ���ӵ�����ͼ��ͼ��ʾ����ͼ�п�֪��Ħ��������_________���� A �ķ���ʽ��___________��
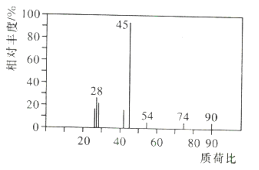
(2)A ���� NaHCO3 ��Һ������Ӧ���� A ���ӵĺ˴Ź���������4�����շ壬�����֮���� 1:1:1:3��A�к��еĹ�����������___________��
(3)д�� A ���ɾۺ���Ļ�ѧ����ʽ___________��
(4)�밴��ϵͳ������д���������� A ��ͬ��ͬ���칹�������___________��
���𰸡� 90g/mol C3H6O3 �ǻ����Ȼ� 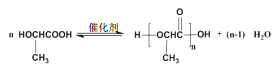 3-�ǻ�����
3-�ǻ�����
����������1��5.4gˮ�����ʵ���Ϊ![]() n��H��=0.6 mol��13.2g������̼�����ʵ���Ϊ
n��H��=0.6 mol��13.2g������̼�����ʵ���Ϊ![]() ��n��C��=n��CO2��=0.3 mol�����л���9.0g��OԪ��������9.0g-0.6g-0.3��12 g=4.8 g��n��O��=
��n��C��=n��CO2��=0.3 mol�����л���9.0g��OԪ��������9.0g-0.6g-0.3��12 g=4.8 g��n��O��=![]() ����n��C����n��H����n��O��=0.3mol��0.6mol��0.3mol=1��2��1����ʵ��ʽΪCH2O�������ʽΪ��CH2O��n��A����Է�������Ϊ90���ɵ�30n=90����ã�n=3�����л���AΪC3H6O3���ʴ�Ϊ��C3H6O3��
����n��C����n��H����n��O��=0.3mol��0.6mol��0.3mol=1��2��1����ʵ��ʽΪCH2O�������ʽΪ��CH2O��n��A����Է�������Ϊ90���ɵ�30n=90����ã�n=3�����л���AΪC3H6O3���ʴ�Ϊ��C3H6O3��
��2��A����NaHCO3��Һ������Ӧ��Aһ�������Ȼ���-COOH�����л���AΪC3H6O3���˴Ź���������4���壬�����֮����1��1��1��3���������4��Hԭ�ӵ���ĿΪ1��1��1��3�������к���1��-COOH��1��-CH3��1��![]() CH��1��-OH���л���A�Ľṹ��ʽΪCH3CH��OH��COOH���ʴ�Ϊ��CH3CH��OH��COOH�к��еĹ�����Ϊ�ǻ����Ȼ���
CH��1��-OH���л���A�Ľṹ��ʽΪCH3CH��OH��COOH���ʴ�Ϊ��CH3CH��OH��COOH�к��еĹ�����Ϊ�ǻ����Ȼ���
��3���л���A����-OH��-COOH���ۺϷ�Ӧ�õ������ķ�Ӧ����ʽΪ![]() ��(4)�밴��ϵͳ������д���������� A ��ͬ��ͬ���칹�������3-�ǻ�����.
��(4)�밴��ϵͳ������д���������� A ��ͬ��ͬ���칹�������3-�ǻ�����.

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�����Ŀ�����������й�����������Һ��������ȷ����(������Һ���ʱ����仯)
�� | �� | �� | �� | |
pH | 12 | 12 | 2 | 2 |
��Һ | ��ˮ | ����������Һ | ���� | ���� |
A. ���٢��зֱ�����Ȼ�茶���������Һ��pHֵ������
B. �ֱ��������������ˮϡ��100����������Һ��pH����>��
C. ���٢�����Һ�������Ϻ�������Һ������
D. ����Һ������Һ��������������Ϻ�������ҺpH��7
����Ŀ����֪������Ũ��Ϊ0.1mol/L�ļ�����Һ��pH�����±��������й�˵����ȷ���ǣ� ��
���� | pH |
NaF | 7.5 |
Na2CO3 | 11.6 |
NaClO | 9.7 |
NaHCO3 | 8.3 |
A. ͬ�¶�ͬŨ���£�����ǿ������˳��Ϊ��HF��H2CO3��HClO
B. ˮ�ⷽ��ʽ��F-+H2O![]() HF+OH-��ƽ�ⳣ��Ϊ1��10-13
HF+OH-��ƽ�ⳣ��Ϊ1��10-13
C. ��CO2ͨ��0.lmol/LNa2CO3��Һ����Һ�����ԣ�����Һ�У�2c(CO32-)+c(HCO3-)=0.1mol/L
D. �����ʵ�����NaF��HF�����Һ������Ũ�ȴ�С��ϵΪ��c(HF)>c(Na+)>c(F-)>c (H+)>c(OH-)