��Ŀ����
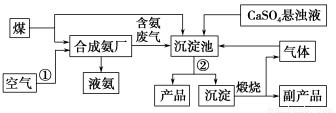
��ҵ��������Ĺ�������ʾ��ͼ���£�
���������գ�
(1)����ˮ���������A��B������(������A��Դ��ʯ��Ҥ��)��д��A��B�Ļ�ѧʽ��
A______________��B____________��
(2)ʵ�����ᴿ���ε�ʵ���������Ϊ��
ȡ����__________��������__________��__________����ȴ�ᾧ��__________����ɡ�
(3)��ҵ��������������У�̼�ữʱ������������__________________��
̼�ữʱû������̼���ƾ��壬��ԭ����______________________��
(4)̼�ữ����ˣ���ҺD����Ҫ�ijɷ���______________(��д��ѧʽ)��������һ�ɷֵ������ӵľ��巽���ǣ�______________________��
(5)��������а���ѭ��ʹ�õģ�Ϊ�ˣ���ҺD����ʯ��ˮ����������ʯ��ˮ���������ķ�Ӧ�����ӷ���ʽΪ��____________________________��
(6)��Ʒ�����к���̼�����ơ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ��__________(ע����ı���ʽ�����õ��йط��ŵĺ���)��
(1)Ca(OH)2��CaO��Na2CO3
(2)�ܽ⡡���ˡ�����������
(3)�о�������(����ֻ���)��̼�����ܽ�ȱ�̼�����ƴ�
(4)NH4Cl��ȡ�����������������ữ������������Һ�а�ɫ����������������������
(5)NH4����OH�� NH3����H2O
NH3����H2O
(6)w(NaHCO3)��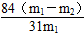 (m1��ʾ��Ӧǰ��Ʒ������m2��ʾ���Ⱥ���������)
(m1��ʾ��Ӧǰ��Ʒ������m2��ʾ���Ⱥ���������)
��������(1)���ݳ�����A����Դ����֪A������CaO��Ca(OH)2��B��������������ȥ��������ij�������Ϊ�������µ�������ѡ��Na2CO3��
(2)���鿼���Դ����ᴿ�Ļ������ܲ�����ȡ�����ܽ�����������������������ȴ���ᾧ����������ɡ�
(3)��������Ȼ�����Һ��ͨ��CO2�ữ�����ڰ��������»����̼�����ƣ�NaCl��NH3��H2O��CO2=NaHCO3����NH4Cl������NaHCO3���ܽ�Ƚ�С������Һ��������
(4)��Һ�к���NH4Cl��Cl���ļ���ͨ������HNO3�ữ��AgNO3��Һ�����ܲ�����ɫ����������֤��Cl���Ĵ��ڡ�
(5)���ӷ���ʽ��NH4�� NH3����H2O��
NH3����H2O��
(6)����Ʒ����Ϊm1�����Ⱥ��������Ϊm2��
2NaHCO3 Na2CO3��CO2����H2O����m
Na2CO3��CO2����H2O����m
168 62
x m1��m2
x��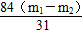
��NaHCO3��������������(NaHCO3)��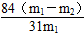 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
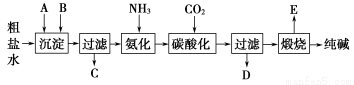
Сѧ��10����Ӧ����ϵ�д�����������Ϣ��ҵ����Ҫ�Ļ������ϡ�ͨ���ý�̿�ڸ����»�ԭ���������Ƶôֹ�(������������������)���ֹ���������Ӧ������������(��Ӧ�¶�450��500 ��)�����Ȼ��辭�ᴿ����������ԭ�ɵøߴ��衣������ʵ�����Ʊ����Ȼ����װ��ʾ��ͼ��
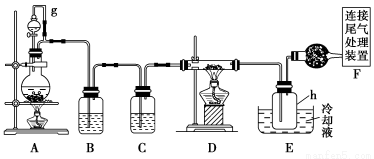
�����Ϣ���£�
a�����Ȼ�����ˮ����ˮ�⣻
b���������������ڸ����¾���������ֱ�ӷ�Ӧ������Ӧ���Ȼ��
c���й����ʵ������������±���
��� | SiCl4 | BCl3 | AlCl3 | FeCl3 | PCl5 |
�е�/�� | 57.7 | 12.8 | �� | 315 | �� |
�۵�/�� | ��70.0 | ��107.2 | �� | �� | �� |
�����¶�/�� | �� | �� | 180 | 300 | 162 |
��ش��������⣺
(1)д��װ��A�з�����Ӧ�����ӷ���ʽ ____________________________��
(2)װ��A��g�ܵ�������________��װ��C�е��Լ���________��װ��E�е�hƿ��Ҫ��ȴ��������________________________________________��
(3)װ��E��hƿ�ռ����Ĵֲ����ͨ������(���ƶ������)�õ��ߴ������Ȼ��裬�����IJ������У�����Ԫ������ܻ����е�����Ԫ����________(��дԪ�ط���)��
(4)Ϊ�˷�������������Ԫ�صĺ������Ƚ�������Ԥ������ʹ��Ԫ�ػ�ԭ��Fe2��������KMnO4����Һ�����������½���������ԭ�ζ�����Ӧ�����ӷ���ʽ�ǣ�5Fe2����MnO4����8H��=5Fe3����Mn2����4H2O
�ζ�ǰ�Ƿ�Ҫ�μ�ָʾ����________(����������������)����˵������______________________��
��ijͬѧ��ȡ5.000 g�������Ԥ������������ƿ�����Ƴ�100 mL��Һ����ȡ25.00 mL������Һ����1.000��10��2 mol��L��1 KMnO4����Һ�ζ����ﵽ�ζ��յ�ʱ�����ı���Һ20.00 mL�������������Ԫ�ص�����������________��