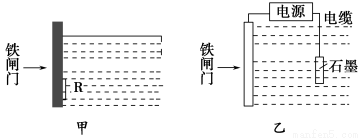
��Ŀ����
����̼������ʱ�У�Ҳ�ǻ���Ҫ������̼���ڹ�ҵ�����������ش������ԭ���ϣ����ŷŻ�����ŷ�������������ͬ�������������ȶ��Ǻܺõ�����̼��������ʽ�������Ǽ����������÷���������(Һ)����������������淋Ĺ��գ�
��ش��������⣺
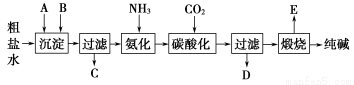
(1)���ղ����������ֱ�Ϊ__________��__________��
(2)д����������Ʒ�������ӷ���ʽ_________________________________��
(3)����ϳɰ��������к��а����ķ�����___________________________
___________________________________________________________��
(4)����Ʒ�Ļ�ѧʽΪ__________�����������������п�ѭ��ʹ�õ�������
________��
(1)Һ��������
(2)CaSO4��2NH3��CO2��H2O=CaCO3����2NH4����SO42��
(3)��ʪ��ĺ�ɫʯ����ֽ���ڹܿڴ�������ֽ������֤����������NH3
(4)CaO��CO2
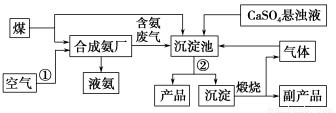
��������(1)�ϳɰ�ԭ��֮һ������Դ�ڿ��������뷽���ǽ�����Һ�������������O2���N2�����������еĻ���ᆳ���˿ɵõ���Ʒ�ͳ�����
(2)����Ʒ����(NH4)2SO4����Ӧ����CaSO4����Һ��CO2��NH3�ȣ������(NH4)2SO4���CaCO3���������ӷ�����CaSO4����ҺҪд����ʽ��
(3)����ʪ��ĺ�ɫʯ����ֽ����NH3��
(4)CaCO3���տ�����CO2��CaO������CO2��ѭ��ʹ�á�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�