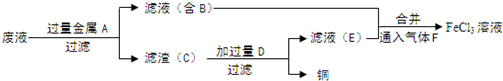
��Ŀ����
19��ij�л���A�������ܶ���ͬ�¡�ͬѹ�������ܶȵ�31����Ϊ��һ���ⶨA �Ļ�ѧʽ����ȡ6.2g A��ȫȼ�գ��õ�������̼��ˮ������ �������Ⱥ�ͨ��������Ũ����ͼ�ʯ�ң����߷ֱ�����5.4g��8.8g������ÿ����Ӧ��������ȫ������1�����л������Է�������Ϊ62��ʵ��ʽ��CH3O������ʽ��C2H6O2��
��2�����������ʾ�С�C-C�����͡�O-H�����������գ����˴Ź�������ֻ��2�����շ��ҷ����֮��Ϊ1��2���ƶϸ��л���Ľṹ��ʽ��HOCH2CH2OH��
��3�����л���������Ʒ�Ӧ�Ļ�ѧ����ʽ��HOCH2CH2OH+2Na��NaOCH2CH2ONa+H2����
���� ��1����ͬ�����£��ܶ�֮�ȵ�����Է�������֮�ȣ������������ܶȼ����л���A����Է���������
Ũ��������5.4gΪˮ����������ʯ������8.8gΪ������̼������������ԭ���غ����6.2gA��C��Hԭ�����ʵ������ٸ��������غ�����ж�6.2g�л���A��Oԭ�����ʵ���������ȷ�����ʽ�������Է�������ȷ������ʽ��
��2�����������ʾ�С�C-C�����͡�O-H�����������գ��˴Ź�������ֻ��2�����շ��ҷ����֮��Ϊ1��2����Ϸ���ʽȷ���ṹ��ʽ��
��3�����д��ǻ�����Na��Ӧ�����л�������������
��� �⣺��1���л���A�������ܶ���ͬ�¡�ͬѹ�������ܶȵ�31������Mr��A��=31��2=62��
Ũ��������5.4gΪˮ�������������ʵ���Ϊ$\frac{5.4g}{18g/mol}$=0.3mol��n��H��=0.6mol��m��H��=0.6mol��1g/mol=0.6g����ʯ������8.8gΪ������̼�������������ʵ���Ϊ$\frac{8.8g}{44g/mol}$=0.2mol��n��C��=0.2mol��m��C��=0.2mol��12g/mol=2.4g�����л�����m��O��=6.2g-0.6g-2.4g=3.2g����n��O��=$\frac{3.2g}{16g/mol}$=0.2mol���ʸ��л������ʽΪCH3O���л�����Է�������Ϊ62�����л������ʽΪC2H6O2��
�ʴ�Ϊ��42��CH3O��C2H6O2��
��2�����������ʾ�С�C-C�����͡�O-H�����������գ��˴Ź�������ֻ��2�����շ��ҷ����֮��Ϊ1��2���л���A�Ľṹ��ʽΪ��HOCH2CH2OH��
�ʴ�Ϊ��HOCH2CH2OH��
��3�����д��ǻ�����Na��Ӧ�����л���������������Ӧ����ʽΪ��HOCH2CH2OH+2Na��NaOCH2CH2ONa+H2����
�ʴ�Ϊ��HOCH2CH2OH+2Na��NaOCH2CH2ONa+H2����
���� �����л������ʽȷ�����㡢ͬ���칹�塢�л�������ʵȣ�����ԭ���غ����ȷ�����ʽ�ǹؼ����ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ���� | B�� | ���� | C�� | ��ά�� | D�� | ������ |
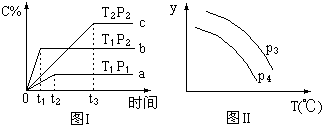 ��ij�ݻ�һ�����ܱ������У������еĿ��淴Ӧ����g��+B��g��?xC��g��������Ӧ���ȣ���ͼ����ʾ�ķ�Ӧ���ߣ����ж϶�ͼ���˵������ȷ���ǣ�T��ʾ�¶ȣ�P��ʾѹǿ��C%��ʾC�������������������
��ij�ݻ�һ�����ܱ������У������еĿ��淴Ӧ����g��+B��g��?xC��g��������Ӧ���ȣ���ͼ����ʾ�ķ�Ӧ���ߣ����ж϶�ͼ���˵������ȷ���ǣ�T��ʾ�¶ȣ�P��ʾѹǿ��C%��ʾC�������������������| A�� | P3��P4��y���ʾA�����ʵ��� | |
| B�� | P3��P4��y���ʾB��������� | |
| C�� | P3��P4��y���ʾ���������ܶ� | |
| D�� | P3��P4��y���ʾ��������ƽ����Է������� |
| ���� | HA | HB | H2C |
| ����ƽ�ⳣ�� ��25�棩 | K1=1.77��10-4 | K1=4.9��10-10 | K1=4.3��10-7 K2=5.6��10-11 |
| A�� | B-+HA��HB+A- | |
| B�� | 2B��+H2C��2HB+C2- | |
| C�� | �к͵��������pH��HA��HB����NaOH����ǰ��С�ں��� | |
| D�� | ���������Ũ�ȵ�NaA��NaB��Һ��������������ǰ�ߴ��ں��� |
| A�� | 150 mL | B�� | 200 mL | C�� | 300 mL | D�� | 400 mL |
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| A | ���������Ӳ㣬K��M�������֮�͵���L������� |
| B | �������н�������ǿ |
| C | �����µ���Ϊ˫ԭ�ӷ��ӣ��⻯���ˮ��Һ�ʼ��� |
| D | Ԫ�����������+7�� |
��AԪ�������ڱ��е�λ�õ������ڵڢ�A�壮
���õ���ʽ��ʾA��BԪ����ɵĻ�������γɹ���
 ��
����2��Ԫ��D��Ԫ��A��ȣ�D�ķǽ����Խ�ǿ�����б�����֤����һ��ʵ����ad����ѡ����ţ���
a��D���⻯���A���⻯���ȶ�
b��������D�ĵ��ʺ�A�ĵ���״̬��ͬ
c��һ��������D��A�ĵ��ʶ������Ʒ�Ӧ
d��D������������Ӧ��ˮ��������ǿ��A������������Ӧ��ˮ���������
��3��X����A��B��C��D����Ԫ���е�ij��Ԫ���γɵĵ��ʣ��ܾ���ͼ��ʾ�Ĺ���ת��ΪW������������ȥ����
X$\stackrel{{O}_{2}}{��}$Y$\stackrel{{O}_{2}}{��}$Z$\stackrel{{H}_{2}O}{��}$W
����Z�Ǻ���ɫ���壬�� Z $\stackrel{H_{2}O}{��}$ W �Ļ�ѧ����ʽΪ3NO2+H2O=2HNO3+NO���˷�Ӧ���������뻹ԭ�������ʵ���֮����1��2��
����Y���д̼�����ζ����ɫ���壬��Yͨ��BaCl2��Һ�У��ٵμ�����H2O2 ��Һ���а�ɫ�������ɣ����ɸð�ɫ�����Ļ�ѧ����ʽΪBaCl2+SO2+H2O2=BaSO4��+2HCl��
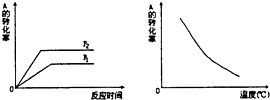 ��ͼ��ʾ�����淴Ӧ��mA��g��+nB��g��?xC��g�����ڲ�ͬ�¶ȡ�ѹǿ�·�Ӧ��A��ת���ʵı仯��������ж��ڷ�Ӧ����ЧӦ�ͷ�Ӧ����ʽ��A��B��C��ϵ�����ж��У���ȷ���ǣ�������
��ͼ��ʾ�����淴Ӧ��mA��g��+nB��g��?xC��g�����ڲ�ͬ�¶ȡ�ѹǿ�·�Ӧ��A��ת���ʵı仯��������ж��ڷ�Ӧ����ЧӦ�ͷ�Ӧ����ʽ��A��B��C��ϵ�����ж��У���ȷ���ǣ�������| A�� | ���ȣ�m+n��x | B�� | ���ȣ�m+n��x | C�� | ���ȣ�m+n��x | D�� | ���ȣ�m+n��x |