��Ŀ����
���и���ʵ��������������ó��Ľ����У���ȷ����
ѡ�� ʵ����������� ʵ�����
A �����ݵ�������Һ�зֱ�μ�NaCl��Һ��
CuSO4��Һ�����й������� �����ʾ������˱���
B ȡ����Fe��NO3��2������ˮ�ܽ⣬��ϡ�����ữ��
�Ρ���KSCN��Һ����Һ��Ϊ��ɫ ��Fe��NO3��2�����Ѿ�
����
C ������ij���ʵ���Һ�μӵ����Ƶ�������Һ�У�
ˮԡ���Ⱥ����������� ������һ������ȩ��
D ͬ�����£��ֱ�0.1mol��L��1������ʹ����
�е�����ʵ�飬����ᴮ���ĵ��ݽϰ� ����������
D
��������
��������� ������Һ�μ�NaCl��Һ���й���������ԭ���Ƿ�������������A�����Fe��NO3��2��NO3‾������ϡ�������Һ�ֺ��д���H+������Һ����HNO3, HNO3���Fe��NO3��2����Ϊ Fe��NO3��2������˵��ԭ�������ʣ���B�����������Ҳ�ܷ���������Ӧ���������Dz���ȩ����C�������ͬŨ�ȵ�����ʹ�����ͬ�����½��е�����ʵ�飬����ᴮ���ĵ��ݽϰ���˵���������̶�С��Ϊ���ᣬ��D����ȷ��
���㣺 ���⿼�鵰���ʡ�����������ԡ�������Ӧ���������Һ�ĵ����ԡ�



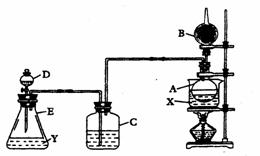 ��.װ������װ���Լ��ǣ���Aƿװ��ˮ�Ҵ����ڷ���ˮ��X����B�������װ��ʯ�ң�
��.װ������װ���Լ��ǣ���Aƿװ��ˮ�Ҵ����ڷ���ˮ��X����B�������װ��ʯ�ң�