��Ŀ����
��֪��C2H5OH+HBr��C2H5Br+H2O��Ϊ֤���Ҵ������к�����ԭ�ӣ��ֲ���һ��װ�ý���ʵ�顣�Ը�������װ��ͼ���Լ���ʵ�����ش��й����⡣
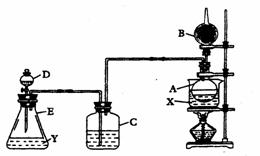 ��.װ������װ���Լ��ǣ���Aƿװ��ˮ�Ҵ����ڷ���ˮ��X����B�������װ��ʯ�ң�
��.װ������װ���Լ��ǣ���Aƿװ��ˮ�Ҵ����ڷ���ˮ��X����B�������װ��ʯ�ң�
��C��D�ж�װŨ�����Eƿ��װ���Լ�Y
ʵ������������ǣ�
��.��ˮԡ����Aƿ����D��Ũ���Ỻ������E�����Լ�Y���ã�����C�е����д������ݷų���Aƿ��X��ɫ����B�ܿڻӷ�������ɵ�ȼ����ش����и����⣺
��1��Eƿ����װ���Լ�Y�����µ�???????________
A.����ʳ��ˮ�������� �� B.MnO2��NaCl�Ļ����������� C.Ũ����
��2��Dƿ��Ũ���������������_____�������� __ ;Cƿ��Ũ���������������________ ___�������������� ___
��3��Aƿ�з�����Ӧ�Ļ�ѧ����ʽ��________________________________����Ӧ������____________�������ɵ�__________��д���ƣ���B���ڴ���ȼ��
��4����ˮ��X��ѡ��_________������ָʾ���õ�ԭ����__���������������� ______
��5����ʵ����֤���Ҵ������к�����ԭ�ӵ�������__________________________________
(6)�����װ���е�Cƿȡ����ʵ��Ŀ���Ƿ��ܴﵽ________����Ϊ_____������ ___������������������������������ ����
(1)C
(2)����Ũ�����е�ˮ�֣��ų�������ʹHCl�����ݳ� ����HCl����
![]()

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���֪��C2H5OH(g)+3O2(g) ="==" 2CO2(g)+3H2O(g) ��H1��-Q1kJ��mol-1��C2H5OH(g) ="==" C2H5OH(l) ��H2��-Q2kJ��mol-1��H2O(g) ="==" H2O(l) ��H3��-Q3kJ��mol-1����ʹ23gҺ��ƾ���ȫȼ�գ��ָ������£���ų�������Ϊ �� ��
| A��Q1��Q2��Q3 |
| B��0.5Q1��0.5Q2��1.5Q3 |
| C��0.5��Q1��Q2��Q3�� |
| D��0.5Q1��1.5Q2��0.5Q3 |


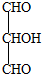


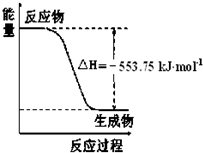 ��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵���
��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵���