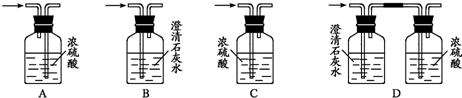
��Ŀ����
Ϊ��֤���Ҵ������к�����ԭ�ӣ��ֲ���һ��װ�ý���ʵ�顣�Ը�����ͼ��ʾװ��ʾ��ͼ���Լ���ʵ�����ش��й����⡣
��.װ������װ���Լ���?
��Aƿװ��ˮ�Ҵ����ڷ���ˮ��X?
��B�������װ��ʯ��?
��C��D�ж�װŨ����?
��Eƿ��װ���Լ�Y?
��.ʵ�����������?
��ˮԡ����Aƿ����D��Ũ���Ỻ������E�����Լ�Y���ã�����C�е����д�������ð����Aƿ��X��ɫ����B�ܿڻӷ��������ȼ�ա�?
������������⣺?
��1��Eƿ����װ���Լ�Y�ǣ�������?
a.����ʳ��ˮ? b.MnO2��NaCl�Ļ����? c.Ũ����?
��2��D��Ũ���������������____________________��Cƿ��Ũ���������������____________________��
��3��Aƿ�з�����Ӧ�Ļ�ѧ����ʽ��______________________________����Ӧ������__________�������ɵ�__________��д���ƣ���B���ڴ���ȼ��
��4����ˮ��X��ѡ��__________��������ָʾ���õ�ԭ����_________________________ _______________��?
��5����ʵ����֤���Ҵ������к�����ԭ�ӵ�������____________________________��
��6�������װ���е�Cƿȡ����ʵ��Ŀ���Ƿ��ܴﵽ��__________����Ϊ______________________________��
��������ʵ���ԭ�����ô����Ȼ��ⷢ��ȡ����Ӧ����ˮ��ͨ������ˮ�����ɣ����жϴ�������ԭ�ӡ�Eƿ��Ӧ���Ƶ��Ȼ��⣬�����Լ�YӦ����Ũ���ᡣŨ�����������Ϊ�˳���ˮ��������ˮ����ͭ��ˮ��������������ԣ����Կ�������ָʾˮ�����ɡ�
�𰸣���1��c?
��2������Ũ�����е�ˮ�֣�ʹHCl�����ݳ�������HCl����
��3��CH3CH2OH+HCl![]() CH3CH2Cl+H2O��ȡ����Ӧ��������
CH3CH2Cl+H2O��ȡ����Ӧ��������
��4����ˮ����ͭ��ʵ������пɹ۲쵽��ˮ����ͭ�ɰױ�����˵����Ӧ����ˮ����
��5��������ˮ����ͭ������֤���˷�Ӧ��һ����ˮ���ɣ�ˮ�к���Ԫ�أ��������������Ȼ��⣬��ֻ�����Ҵ��ṩ?
��6�����ܡ��Ȼ���ӷ�ʱ����ˮ������������ȥ�����϶�ʹ��ˮ����ͭ������ˮ�Ƿ������Ҵ�