��Ŀ����
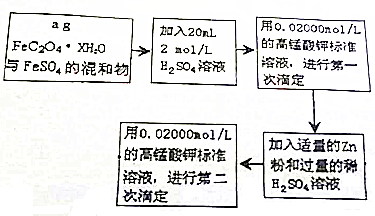
3�� ������Ҫ�Ļ���ԭ�ϣ�ij̽��С���������з�Ӧ��ȡˮ���£�N2H4•H2O����
������Ҫ�Ļ���ԭ�ϣ�ij̽��С���������з�Ӧ��ȡˮ���£�N2H4•H2O����CO��NH2��+2NaOH+NaClO�TNa2CO3+N2H4•H2O+NaCl
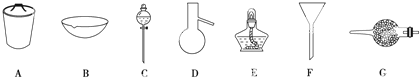
ʵ��һ���Ʊ�NaClO��Һ����ʵ��װ����ͼ1��ʾ��
��1������30%NaOH��Һʱ�����貣����������Ͳ�⣬����BD�����ţ���
A������ƿ B���ձ� C����ƿ D��������
��2����ƿ�з�����Ӧ�Ļ�ѧ����ʽ��Cl2+2NaOH=NaClO+NaCl+H2O��
��3�������ʵ����Ҫ���������к͵ζ�ԭ���ⶨ��Ӧ����ƿ�л����Һ��NaOH��Ũ�ȣ���ѡ�����ṩ���Լ������ʵ�鷽�����ṩ���Լ���H2O2��Һ��FeCl2��Һ��0.1000mol•L-1���ᡢ��̪��Һȡһ������ƿ�ڻ����Һ������������H2O2��Һ�μ�2��3�η�̪��Һ����0.100mol•L-1����ζ����ظ���������2��3�Σ�
ʵ�������ȡˮ���£���ʵ��װ����ͼ2��ʾ��
���Ʒ�Ӧ�¶ȣ�����Һ©������Һ��������������ƿ�У���ַ�Ӧ����������������ƿ�ڵ���Һ���ռ�108��114����֣�
����֪��N2H4•H2O+2NaClO�TN2��+3H2O+2NaCl��
��4����Һ©���е���Һ��B�����ţ���
A��CO��NH2��2��Һ
B��NaOH��NaClO�����Һ
ѡ����������������������Һװ����ƿ�У���Ӧ���ɵ�ˮ���»ᱻ��������������
ʵ�������ⶨ������º�����
��ȡ���5.000g����������NaHCO3���壬��ˮ���250mL��Һ���Ƴ�25.00mL����0.1000mol•L-1��I2��Һ�ζ����ζ������У���Һ��pH������6.5���ң�
����֪��N2H4•H2O+2I2�TN2��+4HI+H2O��
��5���ζ������У�NaHCO3�ܿ�����Һ��pH��6.5���ң�ԭ����NaHCO3����ζ������в�����HI��Ӧ��
��6��ʵ��������I2��Һ��ƽ��ֵΪ18.00mL�������ˮ���£�N2H4•H2O������������Ϊ9%��
���� ��1������һ��������������Һʱ�����岽���Ǽ��㡢�������ܽ⣬NaOH����ʱ��Ҫ�����ձ��гƣ���ȡˮʱ��Ҫ��Ͳ���ܽ�ʱ��Ҫ�ձ�����������
��2������ͨ�뵽ʢ��NaOH����ƿ����NaOH������Ӧ�����Ȼ��ơ��������ƺ�ˮ��
��3���к͵ζ��ⶨ�����Һ��NaOH��Ũ��ʱ����Ҫ�ų���Һ���NaClO�ĸ��ţ���ͨ�����뻹ԭ�ԵĹ������⣬������������������û������Һ�����������ʣ�����ѡ��FeCl2��Һ��ԭ��������������Fe3+������NaOH����Fe��OH��3��������ѡ��ָʾ�������к͵ζ��Ϳ����ˣ�
��4��������ȡˮ���£�N2H4•H2O���ķ�Ӧԭ��Ϊ��CO��NH2��+2NaOH+NaClO=Na2CO3+N2H4•H2O+NaCl����Ϸ�Ӧ����ͷ�Ӧ������жϣ�ˮ���£�N2H4•H2O�����л�ԭ�ԣ��ױ���������������
��5��NaHCO3��ͨ����⻯��ķ�Ӧ������Һ��pH��6.5���ң�
��6����Ϸ�Ӧ������ϵ����õ������ˮ���£�N2H4•H2O��������������
��� �⣺��1������һ��������������Һʱ���ܽ�ʱ��Ҫ�ձ������������ʴ�Ϊ��BD��
��2����ƿ��������NaOH��Ӧ�����Ȼ��ơ��������ƺ�ˮ���ʴ�Ϊ��Cl2+2NaOH=NaClO+NaCl+H2O��
��3���к͵ζ��ⶨ�����Һ��NaOH��Ũ��ʱ����Ҫ�ų���Һ���NaClO�ĸ��ţ���ͨ�����뻹ԭ�Ե�H2O2��ȥNaClO��Ȼ��μӷ�ָ̪ʾ�������ñ�������ζ����NaOH��Һ��Ũ�ȣ��ʴ�Ϊ��ȡһ������ƿ�ڻ����Һ������������H2O2��Һ�μ�2��3�η�̪��Һ���� 0.100mol•L-1����ζ����ظ���������2��3�Σ�
��4����ӦCO��NH2��+2NaOH+NaClO=Na2CO3+N2H4•H2O+NaCl�У�ˮ���£�N2H4•H2O������ԭ�������л�ԭ�ԣ��ױ����������������ʴ�Ϊ��A���������������Һװ����ƿ�У���Ӧ���ɵ�ˮ���»ᱻ��������������
��5��NaHCO3�ܿ�����Һ��pH��6.5���ң�����Ϊ̼�����ƺ͵⻯�ⷴӦ���ʴ�Ϊ��NaHCO3����ζ������в�����HI��Ӧ��
��6����Ϸ�Ӧ������ϵ����õ������ˮ���£�N2H4•H2O��������������
N2H2•H2O+2I2=N2+4HI+H2O
1 2
n 0.1000mol/L��0.018L
n=0.0009mol
250ml��Һ�к��е����ʵ���=0.0009mol��$\frac{250}{25}$=0.009mol
ˮ���£�N2H2•H2O������������=$\frac{0.009��50g/mol}{5.00g}$��100%=9.0%
�ʴ�Ϊ��9%��
���� ���⿼���������Ʊ������ʵ�ʵ���������֤����Ӧ�ã���Ҫ�ǹ��̷����жϣ����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | ���ڵ�ԭ��֮�������ý�����ѧ�� | |
| B�� | �����ͷǽ���Ԫ�ص�����ϣ������γ����Ӽ� | |
| C�� | ��Ԫ�ص�ԭ�Ӱ뾶���������Ӱ뾶�� | |
| D�� | ��Ԫ�ص�ԭ�Ӱ뾶���������Ӱ뾶С |
| A�� | 2.8mol | B�� | 1.6mol | C�� | 3.2mol | D�� | 3.6mol |
| A�� | 2p�������3�����ɶԵ��ӵ�Ԫ�� | |
| B�� | �����������Ǵ�����������3����Ԫ�� | |
| C�� | �γɻ�������������Ԫ�� | |
| D�� | �������������������ڴ�����������Ԫ�� |
| A�� | H2O2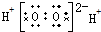 | B�� | MgCl2 | C�� | HClO���� | D�� | CCl4 |