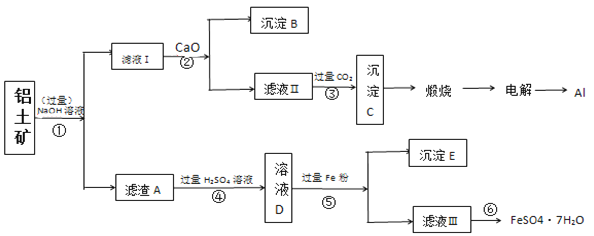
��Ŀ����
����Ŀ��������������־����־�����������й��ڰ�ͭ�ļ��أ���������ͭ��ͭ���Ͻ��������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
(1)��Ԫ�صĻ�̬�۵����Ų�ʽΪ_____________��3d�ܼ��ϵ�δ�ɶԵĵ�����Ϊ______��
(2)���������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ��
��[Ni(NH3)6]SO4�������ӵ����幹����___________��
����[Ni(NH3)6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ______________���ṩ�µ��ӶԵijɼ�ԭ����_____________��
�۰��ķе�_________�������������������������(PH3)��ԭ����__________________������_________���ӣ����������������Ǽ�������������ԭ�ӵĹ���ӻ�����Ϊ_______��
(3)����ͭ����������______________���γɵľ��塣
(4)ij����ͭ�Ͻ�����������ṹ��ͼ��ʾ��
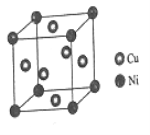
�پ�����ͭԭ������ԭ�ӵ�������Ϊ___________��
�����Ͻ���ܶ�Ϊdg/cm3�������߳�a=______________nm��
���𰸡�3d84s2 2 �������� ��λ�� N ���� NH3���Ӽ���γ���� ���� sp3 ���� 3:1 ![]() ��107
��107
��������
(1)Ni��28��Ԫ�أ���ԭ�Ӻ�����28�����ӣ����ݹ���ԭ����д��Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽ��3d�ܼ��ϵ�δ�ɶԵĵ�����Ϊ2��
(2)��[Ni(NH3)6]SO4�������ӵļ۲���ӶԸ���=4+![]() ���Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ��������ж���������ӵ����幹�ͣ�
���Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ��������ж���������ӵ����幹�ͣ�
�ں��йµ��ӶԺͺ��пչ����ԭ��֮�������λ�����ṩ�µ��ӶԵijɼ�ԭ����Nԭ����
����������⻯���۷е�ϸߣ�
�������ӽṹ���Գƣ�����������IJ��غϣ���������������ԭ��Nԭ�ӵļ۲���ӶԸ���=3+![]() ���Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Nԭ�ӵĹ���ӻ����ͣ�
���Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Nԭ�ӵĹ���ӻ����ͣ�
(3)���������д��ڽ�������
(4)�ٸþ�����Niԭ�Ӹ���=8��![]() =1��Cuԭ�Ӹ���=6��
=1��Cuԭ�Ӹ���=6��![]() =3��
=3��
�ڸþ����Ļ�ѧʽΪCu3Ni�����Ͻ���ܶ�Ϊdg/cm3�����ݾ����ܶȹ�ʽ���㾧��������
(1)Ni��28��Ԫ�أ���ԭ�Ӻ�����28�����ӣ����ݹ���ԭ����д��Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d84s2��[Ar]3d84s2��3d�ܼ��ϵ�δ�ɶԵĵ�����Ϊ2��
��2����[Ni(NH3)6]SO4�������Ӽ۲���ӶԸ���=4+![]() ���Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ��������ж���������ӵ����幹��Ϊ����������
���Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ��������ж���������ӵ����幹��Ϊ����������
�ں��йµ��ӶԺͺ��пչ����ԭ��֮�������λ������[Ni(NH3)6]2+��Ni2+�ṩ�չ����NH3�ṩ�µ��Ӷԣ�������[Ni(NH3)6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ��λ�����ṩ�µ��ӶԵijɼ�ԭ����Nԭ�ӣ�
����������⻯���۷е�ϸߣ������к����������в�����������۷е�������
�������ӽṹ���Գƣ�����������IJ��غϣ�����Ϊ���Է��ӣ���������������ԭ��Nԭ�ӵļ۲���ӶԸ���=3+![]() ���Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Nԭ�ӵĹ���ӻ�����Ϊsp3�ӻ���
���Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Nԭ�ӵĹ���ӻ�����Ϊsp3�ӻ���
(3)���������д��ڽ���������������к��н�������
(4)�ٸþ�����Niԭ�Ӹ���=8��![]() =1��Cuԭ�Ӹ���=6��
=1��Cuԭ�Ӹ���=6��![]() =3����Cu��Niԭ�Ӹ���֮��Ϊ3��1��
=3����Cu��Niԭ�Ӹ���֮��Ϊ3��1��
�ڸþ����Ļ�ѧʽΪCu3Ni�����Ͻ���ܶ�Ϊdg/cm3�����ݾ����ܶȼ���ʽ![]() ��������l=
��������l=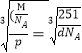 ��107nm��
��107nm��

 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�����Ŀ������˵���Ƿ���ȷ
��1�� | ��2�� | ��3�� | ��4�� |
___ | ___ | ___ | ___ |
��1��22.4LO2��һ������6.02��1023��������
��2����80gNaOH����1Lˮ�У�������Һ��NaOH�����ʵ���Ũ��Ϊ2mol/L
��3��18gH2O�ڱ�״���µ������22.4L
��4���ڱ�״��ʱ��20mLNH3��60mLO2�����ķ��Ӹ�����Ϊ1��3
����Ŀ�����ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ������
Ԫ�ش��� | X | Y | Z | W |
ԭ�Ӱ뾶 | 160 | 143 | 70 | 66 |
��Ҫ���ϼ� |
|
|
|
|
����������ȷ����
A. X�ĵ縺�Դ���Y�ĵ縺��
B. W���⻯���ͬ������Ԫ�ص��⻯���ȶ�������ΪW���⻯���д������
C. Y������������Ӧ��ˮ����������ϡ�����ϡ��ˮ
D. W�ķǽ����Ա�Yǿ