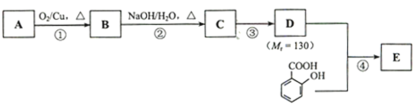
��Ŀ����
����Ŀ���������ı仯�ͷ�Ӧ�Ŀ����ȽǶ��о���Ӧ������Ҫ���塣
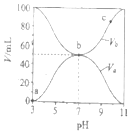
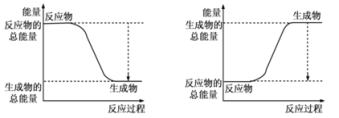
(1)��֪��Ӧ2H2��g��+O2��g��=2H2O��g��Ϊ���ȷ�Ӧ����ͼ����ȷ��ʾ�÷�Ӧ�������仯����_______ ��
A B
��ѧ�� | H��H | O��O | H��O |
����kJ/mol | 436 | 496 | 463 |
�Ӷϼ��ͳɼ��ĽǶȷ���������Ӧ�������ı仯����ѧ���ļ������ϱ���������1molҺ̬ˮ���Էų�����____________kJ
(2)��������ͬ��ͭ����п���õ������Ӻ����CuSO4��Һ�У���Ƴ�ԭ��أ�����������______________, �����ķ�ӦʽΪ______________���������Һ��SO42�� ����______�����������������
(3)һ���¶��£���3 molA�����1mol B����ͨ��һ�ݻ��̶�Ϊ2L���ܱ������У��������·�Ӧ��3A(g)��B(g) ![]() xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ______ ��XΪ______ ������Ӧ��2minʱC��Ũ��______ 0.8mol/L������ڣ�С�ڻ���ڡ�����
xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ______ ��XΪ______ ������Ӧ��2minʱC��Ũ��______ 0.8mol/L������ڣ�С�ڻ���ڡ�����
���𰸡�A 242 Zn Cu2����2e���� Cu �� 0.2mol/(L��min) 2 С��
��������
(1)��ͼ��֪��A�з�Ӧ����������������������������Ϊ���ȷ�Ӧ������2molH2�еĻ�ѧ������2��436kJ����������1molO2�еĻ�ѧ������496kJ������������2��436+496=1368kJ�������γ�4molHO���ͷ�4��463kJ=1852kJ������2molH2��������ȼ������2molˮ�ķ�Ӧ����H=��Ӧ���м���֮���������м���֮��=13682kJ/mol 1852kJ/mol=484kJ/mol����H2��������ȼ������1molˮ�ų�������Ϊ242kJ��
�ʴ�Ϊ��A��242��
(2)п�ϻ��ã�Ϊ��������������ͭΪ�����������Ϸ�����ԭ��Ӧ����ͭ��������ӦʽΪCu2++2e=Cu��
�ʴ�Ϊ��Zn��Cu2++2e=Cu������
(3)��Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L���μӷ�Ӧ��A�����ʵ���Ϊ3mol1.8mol=1.2mol���ɷ���ʽ��֪���μӷ�Ӧ��BΪ1.2mol��![]() =0.4mol����1min�ڣ�B��ƽ����Ӧ����Ϊ
=0.4mol����1min�ڣ�B��ƽ����Ӧ����Ϊ =0.2mol/(L��min)��
=0.2mol/(L��min)��
���ɵ�CΪ0.4mol/L��2L=0.8mol����1.2mol:0.8mol=3:x�����x=2��
���ŷ�Ӧ�Ľ��У���Ӧ������С������Ӧ��2min�ﵽƽ�⣬��1min��ƽ������С��ǰ1min��ƽ�����ʣ�ǰ1min��C��Ũ�ȱ仯Ϊ0.4mol/L�����1min��C��Ũ�ȱ仯С��0.4mol/L����ƽ��ʱC��Ũ��С��0.8mol/L��
�ʴ�Ϊ��0.2mol/(Lmin)��2��С�ڡ�

����Ŀ����H2Sת��Ϊ�������õ���Դ����Դ�о��������Ҫ���⡣
��1��H2S��ת��
�� | ����˹�� |
|
�� | ���������� |
|
�� | ��ֽⷨ |
|
�� ��Ӧ��Ļ�ѧ����ʽ��________��
�� ��Ӧ��____+ 1 H2S ==____Fe2+ + ____S�� + ____������Ӧ������������
�� ��Ӧ��������H2S���ȶ�������H2O�����ԭ�ӽṹ���Ͷ����ȶ��Բ����ԭ��_______��
��2����Ӧ����IJ��ʵͣ���Ӧ���ԭ�������ʵ͡��ҹ�������Ա���뽫������Ӧ��ϣ�ʵ����H2S��Ч����S��H2������ת�ƹ�����ͼ��

���̼ס����У��������ֱ���______��
��3��������ƣ�������Ա�о����¡�
�� �����о��������Ƿ���У�װ����ͼ�������飬n����������Fe3+��p��������H2��n��������Fe3+�Ŀ���ԭ��

����Fe2+ - e- = Fe3+
����2H2O -4e-=O2 +4H+��_______��д���ӷ���ʽ������ȷ�ϣ����Dz���Fe3+��ԭ�����ҿ��С�
�� ���ղ���Fe3+����n����ע��H2S��Һ����S���ɣ���������������p������H2���о�S������ԭ���������ʵ�鷽����______�� ��ȷ�ϣ�S����Fe3+����H2S���ã�H2S����ֱ�ӷŵ硣���̼��С�
��4�����ϣ���Ӧ������ϣ�ͬʱ�ܸ�Ч����H2��S���乤��ԭ����ͼ��

��һ���о����֣�����Fe3+/Fe2+ �⣬I3-/I- Ҳ��ʵ����ͼ��ʾѭ�����̡���ϻ�ѧ���˵��I3-/I- �ܹ�ʹSԴԴ���ϲ�����ԭ��________��