��Ŀ����
����Ŀ��I. X��Y��Z��WΪ��ԭ��������С�������е����ֶ�����Ԫ�ء�X�ɷֱ���Y��W �γ�X2Y��X2Y2��XW�ȹ��ۻ����Z�ɷֱ���Y��W�γ�Z2Y��Z2Y2��ZW�����ӻ����
(1)Z2Y2�ĵ���ʽΪ________��
(2)д��������ZX��ϡ���ᷴӦ�����ӷ���ʽ_______��
(3)�����£�ʵ����0.lmol/L��X2Y2��ˮ��Һ��pH=5.4����X2Y2��ˮ�еĵ��뷽��ʽΪ_______��
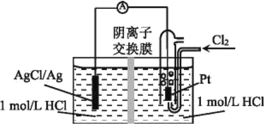
II.���õ�ⱥ��ZW��Һ��CuCl ��Һ�ϳ�1�� 2�����������ʵ��װ����ͼ��ʾ��
(4)���ӽ���ĤXΪ_________(ѡ���������������������ӽ���Ĥ����װ���ܷ�Ӧ�Ļ�ѧ����ʽΪ_______��
(5)Ҫ����1 mol ClCH2CH2Cl�����ͷų���H2�ڱ�״���µ����Ϊ________L��
���𰸡�![]()
![]()
![]() ��
�� ![]() 22.4
22.4
��������
X��Y��Z��WΪ��ԭ��������С�������е����ֶ�����Ԫ�أ�Z�ɷֱ���Y��W�γ�Z2Y��Z2Y2��ZW�����ӻ�������γ�Z2Y��Z2Y2�����ӻ�������Na��OԪ�أ���Z��ԭ����������Y������Y��OԪ�ء�Z��NaԪ�أ�Z��W���γ�ZW�ͻ������W��ԭ����������Z������W��ClԪ�أ�X�ɷֱ���Y��W�γ�X2Y��X2Y2��XW�ȹ��ۻ������X�Ƿǽ���Ԫ�أ���Ϊ��IA��Ԫ�أ���X��HԪ�أ��Դ˽��
��������������֪��XΪH��YΪO��ZΪNa��WΪCl��
I����1��Z2Y2ΪNa2O2��Na2O2Ϊ���ӻ���������ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
��2��ZXΪNaH������HΪ-1�ۣ���ϡ���ᷴӦ���������ơ������������ӷ���ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
��3��X2Y2ΪH2O2��������0.1mol/L H2O2��pH=5.4��˵��H2O2��ˮ�е���Ϊ����ȫ���룬����뷽��ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
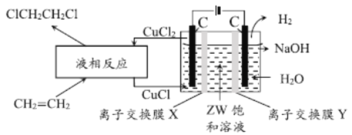
II����4��ZWΪNaCl�������֪��������������������Ӧ��Cu+-e-=Cu2+����Һ����������ӣ�Ϊƽ���ɣ�NaCl��Һ��Cl-ͨ�����ӽ���ĤX���ƣ������ӽ���ĤXΪ�����ӽ���Ĥ����װ�������ʿ�֪����װ�÷�Ӧ��ΪCH2=CH2��H2O��NaCl��������ΪClCH2CH2Cl��H2��NaOH�����ݻ��ϼ������غ��Լ�ԭ���غ��֪�ܷ�ӦΪ��![]() ��
��
�ʴ�Ϊ������![]() ��
��
��5��CH2=CH2��C�Ļ��ϼ�Ϊ-2�ۣ�������ClCH2CH2Cl��C�Ļ��ϼ�Ϊ-1�ۣ�ÿ����1molClCH2CH2Cl��ת�Ƶ��ӵ����ʵ���Ϊ2mol����������������Ӧ��2H++2e-=H2������ÿת��2mol���ӣ�����1molH2�����ڱ���µ����Ϊ1mol��22.4L/mol=22.4L��
�ʴ�Ϊ��22.4��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���״���һ����;�㷺�Ļ���ԭ��,Ҳ����Ϊȼ�ϡ���ش��������⣺
��1����ҵ�ϳ������з�Ӧ�Ʊ��״���CO(g) + 2H2(g) ![]() CH3OH(g) ��H= ��90.1KJmol-1���÷�Ӧ�Է���������________��
CH3OH(g) ��H= ��90.1KJmol-1���÷�Ӧ�Է���������________��
��2��ʵ����ģ����CO��H2��Ӧ���Ƽ״�����250���£���һ������CO��H2Ͷ��2L���ܱ������У������ʵ����ʵ�����mol���仯���±���ʾ����ǰ6minû�иı�������
2min | 4min | 6min | 8min | 20min | �� | |
CO | 0.7 | 0.5 | 0.5 | 0.4 | 0.2 | �� |
H2 | 1.4 | 1 | 1 | 1.8 | 2 | �� |
CH3OH | 0.3 | 0.5 | 0.5 | 0.6 | 0.8 | �� |
�ٴ�0min-4min���ʱ��ķ�Ӧ����v��CO��=_________
��250��ʱ�÷�Ӧƽ�ⳣ��K��ֵΪ________��
������6minʱֻ�ı���һ�������������ı��������________��
������Ӧ�����б����¶Ȳ��䣬��20minʱ,�÷�Ӧ��v��______v����(���� > ������ < ������ = ��)
��3������һ���ݵ��ܱ�������ͨ�����ʵ���֮��Ϊ1��1��CO��H2������CH3OH��������ͬ�¶��£���Ӧ��ͬʱ��ʱCO������������¶ȱ仯��ͼ��_______________

����Ŀ��һ�������£���ӦCO(g)+H2O (g)![]() CO2(g)+H2(g)����H>0���ﵽƽ��״̬�ֱ�ı�ij�������������ĸ�˵���У������ı䡢Ӱ��ԭ��Ӱ��������ȷ����
CO2(g)+H2(g)����H>0���ﵽƽ��״̬�ֱ�ı�ij�������������ĸ�˵���У������ı䡢Ӱ��ԭ��Ӱ��������ȷ����
�����ı� | Ӱ��ԭ�� | Ӱ���� | |
A | �ʵ���С�������ݻ� | �������ռ�ٷֱ��������淴Ӧ���ʾ�ͬ�ȳ̶ȼӿ� | ƽ�ⲻ�ƶ� |
B | �ʵ������¶� | �������ռ�ٷֱ��������淴Ӧ���ʼӿ죬�淴Ӧ���ʼ��� | ƽ�������ƶ� |
C | ������� | �����������������ռ�ٷֱ��������淴Ӧ���ʾ�ͬ�ȳ̶ȼӿ� | ƽ�ⲻ�ƶ� |
D | �ʵ������������ݻ� | �������ռ�ٷֱȲ��䣬���ǵ�λ����ڵĻ���������٣����淴Ӧ����ͬ�ȳ̶ȼ��� | ƽ�ⲻ�ƶ� |
A.AB.BC.CD.D
����Ŀ����գ�
I. ij�л�����C��H��O����Ԫ����ɣ����ģ����ͼ��ʾ��
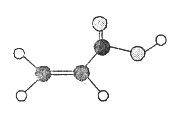
��1�����л���ķ���ʽ��_____________��
��2�����л������������ŵ�������_____________��
��3�����л�����Է����Ӿ۷�Ӧ�������Ľṹ��ʽ��_____________��
��4�������йظ��л���������У���ȷ����_____________������ţ���
a. ����NaHCO3��Һ��Ӧ b. �ܷ���ˮ�ⷴӦ
c. ���������CCl4��Һ��Ӧ d. �������Ը��������Һ��Ӧ
II. ʵ�����Ƶ������г��������ʣ�Ӱ�������ʼ��顣��ͼAΪ����װ�ã�BΪ���ʼ���װ�ã�������б���

��� | ���� | ��Ӧԭ�� | A���Լ� |
�� | ��ϩ | �������NaOH�Ĵ���Һ���� | ______________ |
�� | ��ϩ | ��ˮ�Ҵ���Ũ����������¼�����170�淴Ӧ�Ļ�ѧ����ʽ��__________ | ______________ |
�� | ��Ȳ | ��ʯ�뱥��ʳ��ˮ��Ӧ | ______________ |