��Ŀ����
̼���������ĵ��ʼ��仯�����ڹ�ũҵ����������������Ҫ�����á�
��1�����̼�Ȼ�ԭ���Ȼ�����ʵ���������Ʊ�������������ص��Ȼ�ѧ����ʽ���£�
2Al2O3(s)��2AlCl3(g)��6C(s)=6AlCl(g)��6CO(g) ��H��a kJ��mol��1
3AlCl(g) ��2Al(l)��AlCl3(g) ��H��b kJ��mol��1
��ӦAl2O3(s)��3C(s)��2Al(l)��3CO(g)�ġ�H= kJ��mol��1���ú�a��b�Ĵ���ʽ��ʾ����
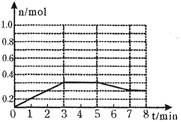
��2���û���̿��ԭ�����Դ����������ij�о�С����ij�ܱ������м���һ�����Ļ���̿��NO��������ӦC(s)+2NO(g) N2(g)+CO2(g) ��H="Q" kJ��mol��1��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)+CO2(g) ��H="Q" kJ��mol��1��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
| ʱ�䣨mol/L�� Ũ�ȣ�mol/L�� | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 1.00 | 0.68 | 0.50 | 0.50 | 0.60 | 0.60 |
| N2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
| CO2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı������������ ������ĸ��ţ���
a��ͨ��һ������NO b������һ�����Ļ���̿
c��������ʵĴ��� d���ʵ���С���������
����30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ3��1��1����Q 0���>����<������
���ں��������£����жϸ÷�Ӧһ���ﵽ��ѧƽ��״̬�������� ����ѡ���ţ���
a����λʱ��������2n mol NO(g)��ͬʱ����n mol CO2(g)
b����Ӧ��ϵ���¶Ȳ��ٷ����ı�
c�����������ܶȲ��ٷ����ı�
d����Ӧ��ϵ��ѹǿ���ٷ����ı�
��3�������������Խ��Al��Ag2O��ؿ�����ˮ�¶�����Դ����ԭ������ͼ��ʾ��
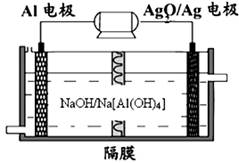
��д���õ��������Ӧʽ �������£��øû�ѧ��Դ�Ͷ��Ե缫���300ml����ͭ��Һ��������������27mg Al���������Һ��pH�� ����������Һ����ı仯����
��1��0.5a��b
��2����0.032mol��L��1��min��1 0.25�� �� ad �ۣ� ��bc
��3��Ag2O+2e��+H2O��2Ag+2OH����2
����

 ��У����ϵ�д�
��У����ϵ�д����Ĺ̶�������;��������̵�����Ȼ�̵���ҵ�̵����������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ���(��������Fe2O3��TiO2)������ˮ������Ӧ�����ɵ���Ҫ����ΪNH3����һ���о�NH3���������¶ȵĹ�ϵ������ʵ�����ݼ��±�(���ա�N2ѹ��1.0��105 Pa����Ӧʱ��1 h)��
| T/K | 303 | 313 | 323 | 353 |
| NH3������/(10��6 mol) | 4.8 | 5.9 | 6.0 | 2.0 |
 4NH3(g)��3O2(g)��H��a kJ��mol��1
4NH3(g)��3O2(g)��H��a kJ��mol��1�ش��������⣺
(1)�˺ϳɷ�Ӧ��a________0����S________0��(�����������������)
(2)��֪��N2(g)��3H2(g)
 2NH3(g)����H����92 .4 kJ��mol��1
2NH3(g)����H����92 .4 kJ��mol��12H2(g)��O2(g) ===2H2O(l)�� ��H ����571.6 kJ��mol��1
��2N2(g)��6H2O(l)===4NH3(g)��3O2 (g)����H��________kJ��mol��1
(3)��323 K��353 K�����������������ٵĿ���ԭ��_________________��
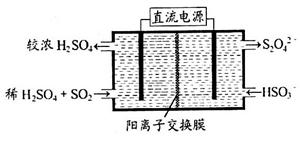
(4)��ҵ�ϳɰ��ķ�ӦΪN2(g)��3H2(g)
 2NH3(g)����H����92 .4 kJ��mol��1���ֱ��о���T1��T2��T3(T1<T2<T3)�����¶��ºϳɰ����Ĺ��ɡ���ͼ�����������¶��²�ͬ��H2��N2����ʼ��ɱ�(��ʼʱN2�����ʵ�����Ϊ1 mol)��N2ƽ��ת���ʵĹ�ϵ����ش�
2NH3(g)����H����92 .4 kJ��mol��1���ֱ��о���T1��T2��T3(T1<T2<T3)�����¶��ºϳɰ����Ĺ��ɡ���ͼ�����������¶��²�ͬ��H2��N2����ʼ��ɱ�(��ʼʱN2�����ʵ�����Ϊ1 mol)��N2ƽ��ת���ʵĹ�ϵ����ش�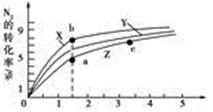
�������������¶��У�����X��Ӧ���¶���________��
��a��b��c����H2��ת������С����________�㡢ת����������________�㡣
�����ݻ�Ϊ1.0 L���ܱ������г���0.30 mol N2(g)��0.80 mol H2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ�������(NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮��)Ϊ4/7���������·�Ӧ2NH3(g)
 N2(g)��3H2(g)��ƽ�ⳣ��Ϊ________ ��
N2(g)��3H2(g)��ƽ�ⳣ��Ϊ________ �� ��Ԫ�صĻ����������������������Ź㷺��Ӧ�á�
��1��400�棬1.01��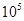 Pa�£��ݻ�Ϊ1��0L���ܱ������г���0.5molSO2, (g)��0.3 molO2 (g)��������Ӧ2SO2(g)��O2(g)
Pa�£��ݻ�Ϊ1��0L���ܱ������г���0.5molSO2, (g)��0.3 molO2 (g)��������Ӧ2SO2(g)��O2(g) 2SO3(g) ��H����198kJ/mol����Ӧ��n(SO3)��n(O2)��ʱ��仯�Ĺ�ϵ����ͼ��ʾ
2SO3(g) ��H����198kJ/mol����Ӧ��n(SO3)��n(O2)��ʱ��仯�Ĺ�ϵ����ͼ��ʾ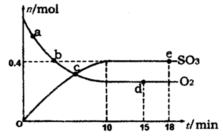 ����Ӧ��ƽ�ⳣ��K��_______��0��10 min����SO2��ʾ��ƽ����Ӧ����_________������ͼ����Ϣ���ж�������������ȷ����_____������ţ���
����Ӧ��ƽ�ⳣ��K��_______��0��10 min����SO2��ʾ��ƽ����Ӧ����_________������ͼ����Ϣ���ж�������������ȷ����_____������ţ���
| A��a��ʱ�̵�����Ӧ���ʱ�b��ʱ�̵Ĵ� |
| B��c��ʱ�̷�Ӧ�ﵽƽ��״̬ |
| C��d���e��ʱ�̵�c(O2)��ͬ |
| D����5 00�棬1.01��105Pa�£���Ӧ�ﵽƽ��ʱ��n( SO3) ��ͼ��e��ʱ�̵�ֵ�� |
��3����ͨ����ⷨʹ��2���е�����Һ������ѭ�����ã��缫��Ϊʯī�缫�����乤��ʾ��ͼ���£�
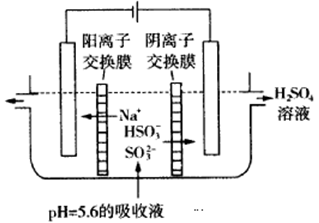
HSO3���������ҷ�Ӧ�ĵ缫��ӦʽΪ________________________�������ҵIJ���_________________��
ijѧ��Ϊ��̽��п�����ᷴӦ�����е����ʱ仯������100mLϡ�����м���������п�ۣ�����ˮ�������ռ���Ӧ�ų�������(���)��ʵ���¼����(�ۼ�ֵ)��
| ʱ��(min) | 1 | 2 | 3 | 4 | 5 |
| �������(mL) | 50 | 120 | 232 | 290 | 310 |
(1)��һʱ���(ָ0��1��1��2��2��3��3��4��4��5 min)��Ӧ������� min��ԭ���� ��
(2)��һ��ʱ�εķ�Ӧ������С min��ԭ���� ��
(3)�����Ӧ̫���ң�Ϊ�˼�����Ӧ���ʶ��ֲ����ٲ����������������������зֱ����������������Һ��A.����ˮ��BNa2SO4��Һ��C.NaNO3��Һ��D.CuSO4��Һ��E.Na2CO3��Һ������Ϊ���е�
�� (��д��ĸ����)��
 2NO���ǵ�������β���к���NO��ԭ��֮һ��
2NO���ǵ�������β���к���NO��ԭ��֮һ��
 CO2(g)��H2(g) ��800��ʱ�����ݻ�Ϊ2.0L���ܱ������г���2.0mol CO(g)��3.0mol H2O(g)�������¶Ȳ��䣬4 min��Ӧ�ﵽƽ�⣬���CO��ת����Ϊ60%��
CO2(g)��H2(g) ��800��ʱ�����ݻ�Ϊ2.0L���ܱ������г���2.0mol CO(g)��3.0mol H2O(g)�������¶Ȳ��䣬4 min��Ӧ�ﵽƽ�⣬���CO��ת����Ϊ60%�� H2(g)+CO2(g)
H2(g)+CO2(g) E(g)
E(g)