��Ŀ����
��֪����1��Al(OH)3�ĵ��뷽��ʽΪ��AlO2��+H++H2O Al(OH)3
Al(OH)3 Al3++3OH��
Al3++3OH��
��2����ˮAlCl3����ķе�Ϊ182.9�棬����ˮ�ĵ��뷽��ʽΪ��AlCl3��Al3����3Cl��
��3��PbSO4������ˮ�������ڴ�������Һ����Ӧ�Ļ�ѧ����ʽΪ��
PbSO4��2CH3COONa��Na2SO4��(CH3COO)2Pb
�����й���Al(OH)3��AlCl3��(CH3COO)2Pb��˵������ȷ����
| A����Ϊǿ����� | B����Ϊ������� |
| C����Ϊ���ӻ����� | D����Ϊ���ۻ����� |
D
����������������ݵ��뷽��ʽ��֪�������������ڵ���ƽ�⣬����������ʡ��Ȼ�������Һ��ȫ�����룬����ǿ����ʡ�PbSO4������ˮ�������ڴ�������Һ����Ӧ�Ļ�ѧ����ʽΪPbSO4��2CH3COONa��Na2SO4��(CH3COO)2Pb���������ӷ�Ӧ������������֪�����������ǦӦ�����ѵ���ģ�������Ǧ��������ʣ����ѡ��A��B�Ǵ���ģ���ˮAlCl3����ķе�Ϊ182.9�棬��˵���û����ﲻ�����ӻ�������������ʹ���Ǧ����������ʣ�Ҳ�������ӻ����������������ʾ��ǹ��ۻ����C����ȷ��D��ȷ����ѡD��
���㣺����ǿ����ʡ�������ʡ����ۻ������Լ����ӻ�������ж�

ij��Һ�д��ڽ϶��H+��SO42-��NO3-������Һ�л����ܴ������ڵ���������
| A��Mg2+��Ba2+��Br- | B��Al3+��CH3COO-��Cl- |
| C��Mg2+��Cl-��Fe2+ | D��Na+��NH4+��Cl- |
���и������ʼ䷴Ӧ���ܰ����ಽ��Ӧ�����ܵ����ӷ���ʽ��ȷ���ǣ� ��
| A��������������ʵ���Ũ�ȵ�������Һ��Ba(OH)2��Һ��ϣ�3Ba2++6OH -+2Al3++3SO42- ="==" 2Al(OH)3��+3BaSO4�� |
| B��FeSO4������Һ��¶�ڿ����У�4Fe2+ + O2 + 2H2O = 4Fe3+ + 4OH - |
| C����AlCl3 ��Һ��Ͷ�����Na��2Al 3+ + 6Na + 6H2O = 2Al(OH)3 �� + 6Na+ + 3H2�� |
| D����FeBr2��Һ��ͨ������Cl2��2Fe2+ + 4Br- + 3Cl2 = 2Fe3+ + 2 Br2 + 6Cl- |
����״̬�����ʣ����ܵ��������ڵ���ʵ���
| A��KCl��Һ | B��Һ̬HCl | C�����ڵ�NaOH | D��������Һ |
�����£����и���������ָ����Һ���ܴ���������� �� ��
| A����ˮ�����c(H+)=10-4mol/L����Һ�У�Fe2+��SO42����K+��NO3�� |
| B����ˮ�����c(H+)=10-14mol/L����Һ�У�Ca2+��Na+��HCO3����NO3�� |
| C��c(H+)/c(OH-)=1012����Һ�У�NH4+��Al3+��SO42����Cl�� |
| D��c(Fe3+)=0.1mol/L����Һ�У�Na+��K+��NO3����SO32�� |
�����������鹲��������ж���ȷ���� �� ��
| A��ʹʯ����Һ������Һ��Fe2����Cl����NO3-��Na���ܴ������� |
| B��ʹ��̪��Һ������Һ��Na����AlO2-��NO3-��HCO3-�ܴ������� |
| C���������۲���H2����Һ�����ܴ��ڴ�����Na����Ba2����AlO2-��NO3- |
| D��������Fe3������Һ��K����NH4+��SO42-��SCN���ܴ������� |
ˮ��ҺX��ֻ��������K����Mg2����Al3����[Al(OH)4]����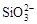 ��
�� ��
��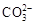 ��
��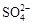 �е����������ӡ�ijͬѧ�Ը���Һ����������ʵ�飺
�е����������ӡ�ijͬѧ�Ը���Һ����������ʵ�飺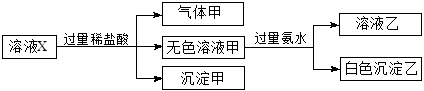
�����ж���ȷ����(����)
| A�������һ���Ǵ����� |
B��K����[Al(OH)4]����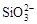 һ����������ҺX�� һ����������ҺX�� |
| C���������ǹ������þ�Ļ���� |
D��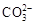 �� ��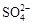 һ������������ҺX�� һ������������ҺX�� |
�����£����и���������ָ����Һ��һ���ܴ���������ǣ�
| A����ʹ����-KI��ֽ����ɫ����Һ�У�K+ SO42- S2- SO32- |
| B������0��1mol��L-1 Fe2+����Һ��Na+Cl- ClO- SO42- |
| C��c��H+��/c��OH-��=1012����Һ�У�Al3+ NH4+ NO3- K+ |
| D������0��1mol��L-1 HCO3-����Һ��Na+ Fe3+ NO3- C6H5O- |
 mol/L��һ���ᷢ����Ӧ����������
mol/L��һ���ᷢ����Ӧ����������