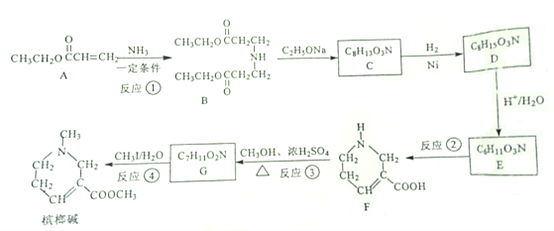
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ
ιΡάΤΦν‘Ύ“ΫΝΤ…œ≥Θ”Ο”Ύ÷ΈΝΤ«ύΙβ―έΘ§Τδ“Μ÷÷Κœ≥…¬ΖœΏ»γœ¬ΘΚ
 “―÷ΣΘΚ
“―÷ΣΘΚ

Θ®1Θ©B÷–Κ§―θΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣ_________ΓΘ
Θ®2Θ©Ζ¥”ΠΔΌΒΡΖ¥”Πάύ–ΆΈΣ________ΓΘ
Θ®3Θ©Ζ¥”ΠΔέΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____________________ΓΘ
Θ®4Θ©CΒΡΫαΙΙΦρ ΫΈΣ__________ΓΘ
Θ®5Θ©Μ·ΚœΈοX «AΒΡΆ§Ζ÷“λΙΙΧεΘ§XΦ»Ρή”κΧΦΥα«βΡΤ»ή“ΚΖ¥”ΠΖ≈≥ωCO2Θ§
”÷Ρή ΙδεΒΡΥΡ¬»Μ·ΧΦ»ή“ΚΆ …ΪΓΘΤδΚΥ¥≈Ι≤’ώ«βΤΉœ‘ Ψ”–4÷÷≤ΜΆ§Μ·ΚœΈοΜΖΨ≥ΒΡ«βΘ§ΖεΟφΜΐ±»ΈΣ3:2:2:1Θ§ ‘–¥≥ωΤδ÷–“Μ÷÷ΖϊΚœ“Σ«σΒΡXΒΡΫαΙΙ
Φρ ΫΘΚ______________ΓΘ
Θ®6Θ©“―÷ΣA‘Ύ«β―θΜ·ΡΤ»ή“Κ÷–Υ°ΫβΒΡ≤ζΈο÷°“Μ «“Μ÷÷–¬–ΆΙΠΡήΗΏΖ÷Ή”≤ΡΝœΘ®PAANaΘ©ΒΡΒΞΧεΘ§–¥≥ω…ζ≥…PAANaΒΡΜ·―ßΖΫ≥Χ Ϋ_________________ΓΘ
Θ®7Θ©ΫαΚœ±ΨΧβ–≈œΔΘ§–¥≥ω“‘““¥ΦΈΣΤπ Φ‘≠Νœ÷Τ±ΗΜ·ΚœΈοCH3COCH2COOCH2CH3ΒΡΚœ≥…¬ΖœΏΘ®ΈόΜζ ‘ΦΝ»Έ―ΓΘ©_________________ΓΘ
ΓΨ¥πΑΗΓΩ θΞΜυ Φ”≥…Ζ¥”Π 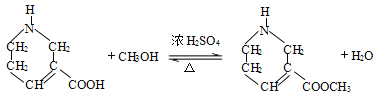
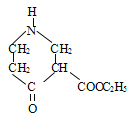 CH2=C(CH3)CH2COOH
CH2=C(CH3)CH2COOH  C2H5OH
C2H5OH![]() CH3CHO
CH3CHO![]() CH3COOH
CH3COOH![]() CH3COOCH2CH3
CH3COOCH2CH3![]() CH3COCH2COOCH2CH3
CH3COCH2COOCH2CH3
ΓΨΫβΈωΓΩ(1). ”…BΒΡΫαΙΙΦρ ΫΩ…÷ΣΘ§B÷–ΒΡΚ§―θΙΌΡήΆ≈ «θΞΜυΘ§Ι ¥πΑΗΈΣΘΚθΞΜυΘΜ
(2). ”…AΚΆBΒΡΫαΙΙΦρ ΫΩ…÷ΣΘ§ΝΫΖ÷Ή”A‘Ύ“ΜΕ®ΧθΦΰœ¬”κΑ±ΤχΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…BΘ§Ι ¥πΑΗΈΣΘΚΦ”≥…Ζ¥”ΠΘΜ
(3). FΖ÷Ή”÷–Κ§”–τ»ΜυΘ§‘Ύ“ΜΕ®ΧθΦΰœ¬”κΦΉ¥ΦΖΔ…ζθΞΜ·Ζ¥”Π…ζ≥…GΘ§GΈΣ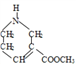 Θ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ
Θ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ ΘΜ
ΘΜ
(4). ΗυΨίΧβ÷–ΧαΙ©ΒΡ“―÷Σ–≈œΔ≤ΔΫαΚœCΒΡΖ÷Ή” ΫΩ…÷ΣΘ§CΒΡΫαΙΙΦρ ΫΈΣ Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ ΘΜ
ΘΜ
(5). Μ·ΚœΈοX «AΒΡΆ§Ζ÷“λΙΙΧεΘ§XΡή”κΧΦΥα«βΡΤ»ή“ΚΖ¥”ΠΖ≈≥ωCO2Θ§ΥΒΟςX÷–Κ§”–τ»ΜυΘ§”÷Ρή ΙδεΒΡΥΡ¬»Μ·ΧΦ»ή“ΚΆ …ΪΘ§ΥΒΟςX÷–Κ§”–ΧΦΧΦΥΪΦϋΘ§ΤδΚΥ¥≈Ι≤’ώ«βΤΉœ‘ Ψ”–4÷÷≤ΜΆ§Μ·ΚœΈοΜΖΨ≥ΒΡ«βΘ§ΖεΟφΜΐ±»ΈΣ3:2:2:1Θ§ΖϊΚœΧθΦΰΒΡ”–ΘΚCH2=C(CH3)CH2COOHΓΔCH2=C(COOH)CH2 CH3Β»Θ§Ι ¥πΑΗΈΣΘΚCH2=C(CH3)CH2COOHΘΜ
(6). AΈΣCH2=CHCOOCH2CH3Θ§‘ΎNaOH»ή“Κ÷–Υ°Ϋβ…ζ≥…CH2=CHCOONaΘ§CH2=CHCOONa÷–Κ§”–ΧΦΧΦΥΪΦϋΘ§Ω…ΖΔ…ζΦ”ΨέΖ¥”ΠΘ§Υυ“‘…ζ≥…PAANaΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ ΘΜ
ΘΜ
(7). ΗυΨίΧβ÷––≈œΔΩ…÷ΣΘ§“‘““¥ΦΈΣΤπ Φ‘≠Νœ÷Τ±ΗΜ·ΚœΈοCH3COCH2COOCH2CH3Θ§Ω…œ»”…““¥ΦΖΔ…ζ¥ΏΜ·―θΜ·…ζ≥…““»©Θ§‘Ό―θΜ·…ζ≥…““ΥαΘ§““Υα”κ““¥ΦΖΔ…ζθΞΜ·Ζ¥”Π…ζ≥…““Υα““θΞΘ§‘Ύ““¥ΦΡΤΉς”Οœ¬Ζ¥”Π…ζ≥…CH3COCH2COOCH2CH3Θ§Κœ≥…¬ΖœΏΈΣΘΚC2H5OH ![]() CH3CHO
CH3CHO ![]() CH3COOH
CH3COOH ![]() CH3COOCH2CH3
CH3COOCH2CH3 ![]() CH3COCH2COOCH2CH3Θ§Ι ¥πΑΗΈΣΘΚC2H5OH
CH3COCH2COOCH2CH3Θ§Ι ¥πΑΗΈΣΘΚC2H5OH ![]() CH3CHO
CH3CHO ![]() CH3COOH
CH3COOH ![]() CH3COOCH2CH3
CH3COOCH2CH3 ![]() CH3COCH2COOCH2CH3ΓΘ
CH3COCH2COOCH2CH3ΓΘ

 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ