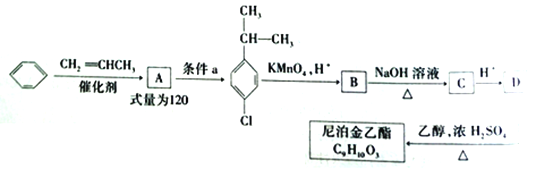
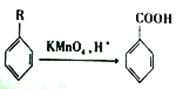
��Ŀ����
����Ŀ����1��4.9 g H2SO4��________________��H2SO4���ӣ��ܺ�__________mol NaOH��ȫ��Ӧ��
��2��������̼ͬԭ������CO��CO2����������Ϊ________________��
��3����ͬ��ͬѹ�£���������CO��NO�������֮��Ϊ___________��
��4����״�����Т�44.8 L CH4����9.03��1023��NH3���ӣ���85 g H2S�������壬����������������ʵ����Ӵ�С��˳����________________ (�����)��
��5����һ�����¶Ⱥ�ѹǿ�£�1�������X2��3�������Y2��������2���������C����û�����Ļ�ѧʽ��____________��
���𰸡�3.01��1022 (��0.05NA) 0.1 7��11 15��14 ���������� XY3 (��Y3X)
��������
��1��4.9 g H2SO4�����ʵ���Ϊ4.9g��98g/mol=0.05mol������������Ӹ���Ϊ0.05 NA���ɷ�Ӧ2NaOH+H2SO4=Na2SO4+2H2O����֪����NaOH�����ʵ���Ϊ0.05mol��2=0.1mol���ʴ�Ϊ��3.01��1022 (��0.05NA)��0.1��
��2��������̼ͬԭ������CO��CO2�������ʵ�����ȣ�����������Ϊ28��44=7:11���ʴ�Ϊ7:11��
��3����ͬ��ͬѹ�£���������CO��NO�������ʵ���֮��Ϊ![]() =15:14�������֮�ȵ��������ʵ���֮�ȣ��ʴ�Ϊ15:14��
=15:14�������֮�ȵ��������ʵ���֮�ȣ��ʴ�Ϊ15:14��
��4����״���¢�44.8 L CH4����9.03��1023��NH3���ӣ���85 g H2S�������壬��������������ʵ����ֱ�Ϊ![]() =2mol��
=2mol��![]() =1.5mol��
=1.5mol��![]() =2.5mol���ʴ�Ϊ���ۡ��١�����
=2.5mol���ʴ�Ϊ���ۡ��١�����
��5�����ݰ����ӵ����ɣ���������֮�ȵ������ʵ���֮�ȣ����ڻ�ѧ����ʽ�ļ�����֮�ȣ��ɵã�X2+3Y2=2C�����ݻ�ѧ��Ӧǰ��ԭ���������Ŀ��ȣ��ɵ���������Ӻ�1��Xԭ�ӣ�3��Yԭ�ӣ���ѧʽΪXY3���ʴ�Ϊ��XY3 (��Y3X)��

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�����Ŀ���Ȼ�������SOCl2����һ��Һ̬������е�Ϊ77������ũҩ����ҩ��ҵ����;�㷺��SOCl2��ˮ���ҷ�Ӧ��Һ���ϲ��������������д̼�����ζ�����������ʵ���Һϳ�ԭ����SO2+Cl2+SCl2�T2SOCl2������װ����ͼ��ʾ���ش��������⣺

��1������c��������________
��2��ʵ������Cl2�Ļ�ѧ����ʽΪ________________________
��3�����������Ʊ� SO2�ķ��������ѡ����_______
���� | �� | �� | �� | �� |
����װ�� |
|
|
|
|
��ѡ�Լ� |
|
|
|
|
��4��d�����߿�����������װ�ã�����������˳��������װ�õ����÷ֱ���_______________��
��5��ʵ�������,��������ƿ�л������뿪�ķ����� _________(��֪SCl2�ķе�Ϊ50��)
��6�������һ����ʵ�鷽����֤H2SO3����ǿ��H2CO3����Ҫ˵��ʵ�鲽�衢����ͽ��ۣ�____________________________________________��������ѡ��
��ѡ���Լ���SO2��NaHCO3������KMnO4��NaHSO3������ˮ������ʯ��ˮ��Ʒ����Һ��pH��ֽ��
��7��Ϊ�ⶨij�����Ŀ����ж�����������̽��С���������ʵ�飺���Թ��м���һ�����ĺ��⣨I2��0.635mg�ĵ���Һ���ټ���2~3�ε�����Һ�����Թ���ͨ�����������Һ����ɫ��Ϊ��ɫʱǡ����ȫ��Ӧ������ȥ�������Ϊ500L����ͨ�������жϳ��˿����ж��������Ũ��_____mg/m3������ѧ����ʽΪ��SO2+I2+2H2O=H2SO4+2HI��