��Ŀ����
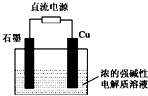
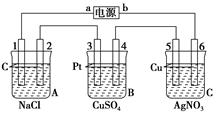
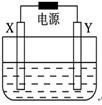
��ͼ��X��Y��Ϊʯī�缫��
(��)�����ҺΪ���з�̪�ı���ʳ��ˮ����ⷴӦ�����ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣ��������Уߣߣߣ�(�����������)��������ֺ�ɫ��
(��)�����ҺΪ500 mL��A���ʵ�ij��ɫ��Һ�����һ��ʱ�䣬�۲쵽X�缫�����к�ɫ�Ĺ�̬�������ɣ�Y�缫����ɫ�������ɣ���Һ��ԭ��������ȫ����ֹͣ��⣬ȡ��X�缫��ϴ�ӡ�����������缫����1.6 g��
(1)������Һ��pHΪ�ߣߣߣ�Ҫʹ������Һ�ָ������ǰ��״̬�������һ�����ģߣߣߣ�(��������ʵĻ�ѧʽ)��(������ǰ����Һ���������)
(2)�����Ʋ�ԭ��Һ��������������ӿ����ǣߣߣߣߣ������ʵ����֤����Ʋ⣬д��ʵ��IJ������衢����ͽ��ۣ�

(��)�����ҺΪ���з�̪�ı���ʳ��ˮ����ⷴӦ�����ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣ��������Уߣߣߣ�(�����������)��������ֺ�ɫ��
(��)�����ҺΪ500 mL��A���ʵ�ij��ɫ��Һ�����һ��ʱ�䣬�۲쵽X�缫�����к�ɫ�Ĺ�̬�������ɣ�Y�缫����ɫ�������ɣ���Һ��ԭ��������ȫ����ֹͣ��⣬ȡ��X�缫��ϴ�ӡ�����������缫����1.6 g��
(1)������Һ��pHΪ�ߣߣߣ�Ҫʹ������Һ�ָ������ǰ��״̬�������һ�����ģߣߣߣ�(��������ʵĻ�ѧʽ)��(������ǰ����Һ���������)
(2)�����Ʋ�ԭ��Һ��������������ӿ����ǣߣߣߣߣ������ʵ����֤����Ʋ⣬д��ʵ��IJ������衢����ͽ��ۣ�
����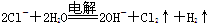 ����
����
(��)(1)1��CuO��CuCO3
(2)��������ӡ�ȡ��������Һ���Թ��У��μ����������������������Ȼ�����Һ�����а�ɫ������������֤������������ӡ�����������ӡ�ȡ��������Һ���Թ��У�����Ũ����μ�Ũ�����ͭ�ۣ����к���ɫ�����������֤������������ӡ�
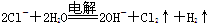 ����
����(��)(1)1��CuO��CuCO3
(2)��������ӡ�ȡ��������Һ���Թ��У��μ����������������������Ȼ�����Һ�����а�ɫ������������֤������������ӡ�����������ӡ�ȡ��������Һ���Թ��У�����Ũ����μ�Ũ�����ͭ�ۣ����к���ɫ�����������֤������������ӡ�
���������(��)��װ��Ϊ��ⱥ��ʳ��ˮ��װ�ã����ⷴӦ�����ӷ���ʽΪ
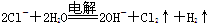 ���ڸõ��ص����������������������������������������������ƣ����Ե���������������������ֺ�ɫ��(��)�����ҺΪ500 mL��A���ʵ�ij��ɫ��Һ�����һ��ʱ�䣬�۲쵽X�缫�����к�ɫ�Ĺ�̬�������ɣ���ú�ɫ����Ϊͭ������ɫ��Һ��ͭ���ӵ���Һ��������Һ��������������ͭ���ʣ���缫��ӦΪ��
���ڸõ��ص����������������������������������������������ƣ����Ե���������������������ֺ�ɫ��(��)�����ҺΪ500 mL��A���ʵ�ij��ɫ��Һ�����һ��ʱ�䣬�۲쵽X�缫�����к�ɫ�Ĺ�̬�������ɣ���ú�ɫ����Ϊͭ������ɫ��Һ��ͭ���ӵ���Һ��������Һ��������������ͭ���ʣ���缫��ӦΪ��Cu2����2e��=Cu,���������������ŵ���������������缫��ӦΪ2H2O��4e��=O2����4H+�����������缫����1.6 g��������ͭ1.6g��ͨ���ĵ�����Ϊ1.6/64��2=0.05mol�����Բ����������ӵ����ʵ���Ϊ0.05mol������c(H+)=0.05/0.5=0.1mol/L������pH=-lg0.1=1��Ҫʹ������Һ�ָ������ǰ��״̬����Ϊ����������Ԫ�غ�ͭԪ�أ����������һ������CuO��CuCO3��(2)ԭ��Һ��������������ӿ�������������ӣ�����֤�Ĺ���Ϊ��ȡ��������Һ���Թ��У��μ����������������������Ȼ�����Һ�����а�ɫ������������֤������������ӡ�������������ӡ�ȡ��������Һ���Թ��У�����Ũ����μ�Ũ�����ͭ�ۣ����к���ɫ�����������֤������������ӡ�
���������⿼���˵��ص�֪ʶ����֪ʶ���Ǹ߿�������ص���ѵ㣬���⿼���֪ʶ�Ƚ�ȫ�棬�����ڿ���ѧ���Ը�֪ʶ����������������Ѷ����С�

��ϰ��ϵ�д�
 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
�����Ŀ
 Cu2O+H2������˵����ȷ����
Cu2O+H2������˵����ȷ����