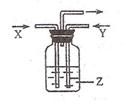
��Ŀ����
��ҵ�ϳ��ð��������������ᣬ����̰������Ĵ�����(����Ϊ����Ͻ�˿��)��һ��������������ˮ���ն��������������ᡣ��ش��������⣺
 (1)���������Ļ�ѧ����ʽΪ ��
(1)���������Ļ�ѧ����ʽΪ ��
 (2)ԭ�����п����������������Ҫԭ���� ��
(2)ԭ�����п����������������Ҫԭ���� ��
 (3)������Ͻ����ɱ�˿������Ҫԭ���� ��
(3)������Ͻ����ɱ�˿������Ҫԭ���� ��
 (4)ˮ���ն���������������Ϊ���ȷ�Ӧ���仯ѧ����ʽΪ ��Ϊ�����ˮ�Զ��������������ʣ��ɲ�ȡ�Ĵ�ʩΪ (��2��)
(4)ˮ���ն���������������Ϊ���ȷ�Ӧ���仯ѧ����ʽΪ ��Ϊ�����ˮ�Զ��������������ʣ��ɲ�ȡ�Ĵ�ʩΪ (��2��)
 (1)���������Ļ�ѧ����ʽΪ ��
(1)���������Ļ�ѧ����ʽΪ �� (2)ԭ�����п����������������Ҫԭ���� ��
(2)ԭ�����п����������������Ҫԭ���� �� (3)������Ͻ����ɱ�˿������Ҫԭ���� ��
(3)������Ͻ����ɱ�˿������Ҫԭ���� �� (4)ˮ���ն���������������Ϊ���ȷ�Ӧ���仯ѧ����ʽΪ ��Ϊ�����ˮ�Զ��������������ʣ��ɲ�ȡ�Ĵ�ʩΪ (��2��)
(4)ˮ���ն���������������Ϊ���ȷ�Ӧ���仯ѧ����ʽΪ ��Ϊ�����ˮ�Զ��������������ʣ��ɲ�ȡ�Ĵ�ʩΪ (��2��)��1��
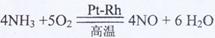
��2����߰���ת���ʺ�һ��������ת����
��3������λ�����Ĵ����뷴Ӧ��ĽӴ����
��4��3NO2 + H2O = 2HNO3 + NO ��ѹ ����
��1��
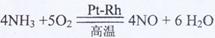
��2������ƽ��ԭ��������Ӧ���Ũ�ȣ�ƽ��������Ӧ�����ƶ��������ԭ�ϵ�ת���ʣ�
��3������Ϊ��������Ͻ�ı������ʹ�Ӵ����������������Ч����
��4��Ϊ����������ʣ��ɸ����ܽ���̷��Ȳ��ý��´���������������տ��ü�ѹ�ķ�ʽ��
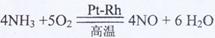
��2������ƽ��ԭ��������Ӧ���Ũ�ȣ�ƽ��������Ӧ�����ƶ��������ԭ�ϵ�ת���ʣ�
��3������Ϊ��������Ͻ�ı������ʹ�Ӵ����������������Ч����
��4��Ϊ����������ʣ��ɸ����ܽ���̷��Ȳ��ý��´���������������տ��ü�ѹ�ķ�ʽ��

��ϰ��ϵ�д�
�����Ŀ