��Ŀ����
(10��)����ʡӵ�кܳ��ĺ����ߣ����зḻ�ĺ�����Դ������λ����DZ�ʡ��һ����Ҫ��ҵ������±�����õ��Ĺ�±ʯ(KCl��MgCl2��6H2O)�����У�������Լ8����NaCl��
Ϊ�˴ӹ�±ʯ����ȡKCl��MgCl2��ijѧϰС��������ϵõ�MgCl2��KCl��NaCl�ڲ�ͬ�¶��µ��ܽ��S(g/100gˮ�����������£�

����Ƴ������ʵ�����̣�
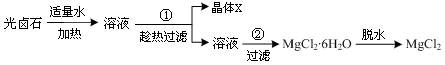
���������ṩ�����ݺ�ʵ��ش��������⣺
����1������ʵ�������о���X�Ļ�ѧʽΪ �����Ƶõ�X�����������Ŀ��������ʣ�����
�ķ�����һ���ᴿ������������г��ȹ��˵�ԭ���ǣ�
����2����ҵ�ϲ��õ����ˮ�Ȼ�þ�ķ�����ȡ����þ��
��1�����ڿ����м���MgCl2��6H2O�����ɵ���Mg(OH)Cl��д����Ӧ�Ļ�ѧ����ʽ��
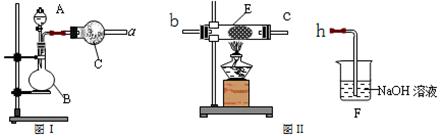
��2����С��ͬѧ��ȡ��Ũ�������Ũ������ȡ�Ȼ�������ķ���������ͼ��ʵ��װ�ã��ڸ����HCl�����м���MgCl2��6H2O���õ���ˮMgCl2��װ����ʢ��Ũ����������ֱ�
�� ����д�����ķ��ţ�����Ҫ���ȵ������� ��ͬ�ϣ���
��3��Ϊ��ֹ�Ի�������Ⱦ������Ҫ��β�����գ���ʵ�������е��������Լ�ֻ�У��ձ������ܡ�����ˮ���ƾ����������Ȼ�̼������Ϊ�������һ��β��������װ�ã���װ��ͼ���ڷ����ڲ���ͼ�б��������Լ���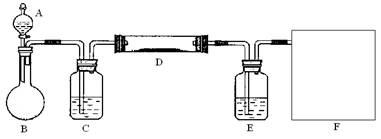
Ϊ�˴ӹ�±ʯ����ȡKCl��MgCl2��ijѧϰС��������ϵõ�MgCl2��KCl��NaCl�ڲ�ͬ�¶��µ��ܽ��S(g/100gˮ�����������£�

����Ƴ������ʵ�����̣�

���������ṩ�����ݺ�ʵ��ش��������⣺
����1������ʵ�������о���X�Ļ�ѧʽΪ �����Ƶõ�X�����������Ŀ��������ʣ�����
�ķ�����һ���ᴿ������������г��ȹ��˵�ԭ���ǣ�
����2����ҵ�ϲ��õ����ˮ�Ȼ�þ�ķ�����ȡ����þ��
��1�����ڿ����м���MgCl2��6H2O�����ɵ���Mg(OH)Cl��д����Ӧ�Ļ�ѧ����ʽ��
��2����С��ͬѧ��ȡ��Ũ�������Ũ������ȡ�Ȼ�������ķ���������ͼ��ʵ��װ�ã��ڸ����HCl�����м���MgCl2��6H2O���õ���ˮMgCl2��װ����ʢ��Ũ����������ֱ�
�� ����д�����ķ��ţ�����Ҫ���ȵ������� ��ͬ�ϣ���
��3��Ϊ��ֹ�Ի�������Ⱦ������Ҫ��β�����գ���ʵ�������е��������Լ�ֻ�У��ձ������ܡ�����ˮ���ƾ����������Ȼ�̼������Ϊ�������һ��β��������װ�ã���װ��ͼ���ڷ����ڲ���ͼ�б��������Լ���

(��10��) ����1�� KCl��1�֣����ؽᾧ��1�֣�����ֹ�Ȼ�þ������1�֣���
����2��(1) MgCl2��6H2O=Mg(OH)Cl��HCl����5H2O����2�֣�
(2)A��C��E��2�֣�������D��1�֣�
(3)��2�֣�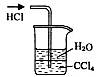
����2��(1) MgCl2��6H2O=Mg(OH)Cl��HCl����5H2O����2�֣�
(2)A��C��E��2�֣�������D��1�֣�
(3)��2�֣�

��

��ϰ��ϵ�д�
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
�����Ŀ


 ����
����