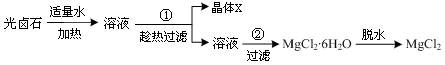
��Ŀ����
��17�֣�ͼ���ǻ�ѧʵ�����г����Ʊ�����������IJ�������װ�á�ijѧУͬѧ������ѧ�����Լ��������������ʵ�顣

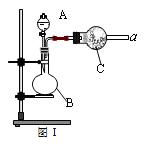
��1��ͼ��������B�����ƣ�_______________________��
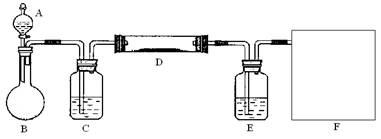
��2����ͬѧ������ͼ��װ���Ʊ����ռ������NO2���壬���ڷ����ڻ����ü���ƿ�ռ�NO2��װ��ͼ����β������װ�ã�����B�з�����Ӧ�����ӷ���ʽΪ________________��
��3��ͬѧ������ͼ��װ��ͨ��������Ӧǰ��C��������ȷ��Na2CO3��NaCl����������Na2CO3��������A�м���ϡ���ᣬB�м���Na2CO3��NaCl�������C�м����ʯ�ҡ���װ�ô��ڽ϶�ȱ�ݣ��Ӷ�����ʵ�����ϴ�����˵�����е�����ȱ�ݣ�
��______________________________________________________________________��
��______________________________________________________________________��
��4��ͬѧ������ͼ��װ����ȡ�����������Ļ�����壬��������ͼ��װ����֤����ijЩ���ʡ�A�м���Ũ��ˮ��C�м����ʯ�ң�E�ڷ��ô���(��ʯ��)������������a��b��c��h���Ӹ�������
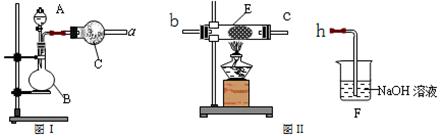
��B�����������Լ�������Ϊ____________________��B���ܲ���������������������ԭ����_______________________________________________________________��
��ʵ���й۲쵽E���к���ɫ������֣�֤����������_________�ԣ�E�з�����Ӧ��������ѧ����ʽΪ��____________________________��2NO + O2 ����2NO2��


��1��ͼ��������B�����ƣ�_______________________��
��2����ͬѧ������ͼ��װ���Ʊ����ռ������NO2���壬���ڷ����ڻ����ü���ƿ�ռ�NO2��װ��ͼ����β������װ�ã�����B�з�����Ӧ�����ӷ���ʽΪ________________��
��3��ͬѧ������ͼ��װ��ͨ��������Ӧǰ��C��������ȷ��Na2CO3��NaCl����������Na2CO3��������A�м���ϡ���ᣬB�м���Na2CO3��NaCl�������C�м����ʯ�ҡ���װ�ô��ڽ϶�ȱ�ݣ��Ӷ�����ʵ�����ϴ�����˵�����е�����ȱ�ݣ�
��______________________________________________________________________��
��______________________________________________________________________��
��4��ͬѧ������ͼ��װ����ȡ�����������Ļ�����壬��������ͼ��װ����֤����ijЩ���ʡ�A�м���Ũ��ˮ��C�м����ʯ�ң�E�ڷ��ô���(��ʯ��)������������a��b��c��h���Ӹ�������

��B�����������Լ�������Ϊ____________________��B���ܲ���������������������ԭ����_______________________________________________________________��
��ʵ���й۲쵽E���к���ɫ������֣�֤����������_________�ԣ�E�з�����Ӧ��������ѧ����ʽΪ��____________________________��2NO + O2 ����2NO2��
��17�֣���1��Բ����ƿ ��1�֣�
��2��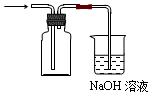 ��2�֣�
��2�֣�
Cu + 4H��+ 2NO3���� Cu2��+ 2NO2��+ 2H2O ��2�֣�
��3��û������Բ����ƿ��ˮ������װ�ã����CO2�л��е�ˮ����Ҳ�ᱻC���գ���������������ͨ�������е�CO2��ˮ����Ҳ�ᱻ���գ���Ӧ������װ���ڲ�����CO2������ȫ�ų����ȵȡ����𰸺��������֣� ��ÿ��2�֣���4�֣�
��4���ٹ������� ��2�֣�����������Ũ��ˮ�е�H2O��Ӧ�����������ƺ�������ͬʱ�ų������ȣ��¶�����ʹ�����ܽ�ȼ�С���ݳ����������Ƶ������OH�������˰�ˮ��OH��Ũ�ȣ���ʹ��ˮ����ƽ�����ƣ����°����ų��� ��2�֣�
�ڻ�ԭ�� ��2�֣�4NH3 + 5O2===== 4NO + 6H2O 2NO + O2 ���� 2NO2 ��2��
��2��
 ��2�֣�
��2�֣� Cu + 4H��+ 2NO3���� Cu2��+ 2NO2��+ 2H2O ��2�֣�
��3��û������Բ����ƿ��ˮ������װ�ã����CO2�л��е�ˮ����Ҳ�ᱻC���գ���������������ͨ�������е�CO2��ˮ����Ҳ�ᱻ���գ���Ӧ������װ���ڲ�����CO2������ȫ�ų����ȵȡ����𰸺��������֣� ��ÿ��2�֣���4�֣�
��4���ٹ������� ��2�֣�����������Ũ��ˮ�е�H2O��Ӧ�����������ƺ�������ͬʱ�ų������ȣ��¶�����ʹ�����ܽ�ȼ�С���ݳ����������Ƶ������OH�������˰�ˮ��OH��Ũ�ȣ���ʹ��ˮ����ƽ�����ƣ����°����ų��� ��2�֣�
�ڻ�ԭ�� ��2�֣�4NH3 + 5O2===== 4NO + 6H2O 2NO + O2 ���� 2NO2 ��2��
��

��ϰ��ϵ�д�
�����Ŀ