��Ŀ����
������FeS2������SΪ-1�ۣ��ǹ�ҵ�����������Ҫԭ�ϣ�FeS2�ڸ�������������Ӧ��3FeS2+8O2
6SO2+Fe3O4
��1������1mol FeS2�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ______��
��2������������SO2����������SO3��2SO2��g��+O2��g��
2SO3��g����H��
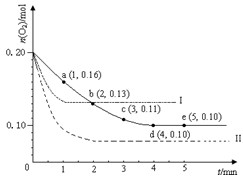
��֪T��ʱ��SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��P���Ĺ�ϵ��ͼ������ͼ�ش��������⣺
��T��ʱ����2.0mol SO2��1.0mol O2����10L�ܱպ��ݷ�Ӧ���У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10MPa������÷�Ӧ��ƽ�ⳣ����д��������̣�������������λ��Ч���֣���______��
��T��ʱ��ƽ����A��䵽B��ʱ��ƽ�ⳣ��K��A��______K��B�������������������=������
����֪1g����������ȫ��Ӧ������̬�������ų�����1.536kJ����������Ӧ�ķ�Ӧ�ȡ�H=______��������С�����һλ��
��3��ij����С������ԭ���ԭ������SO2��O2��H2O���Ʊ����ᣬװ����ͼ���缫A��BΪ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ������ʣ�
��B�缫�ĵ缫��ӦΪ______��
����Һ��H+���ƶ���������______���ص�______���أ���A��B����

| ||
��1������1mol FeS2�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ______��
��2������������SO2����������SO3��2SO2��g��+O2��g��
| ||
��֪T��ʱ��SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��P���Ĺ�ϵ��ͼ������ͼ�ش��������⣺
��T��ʱ����2.0mol SO2��1.0mol O2����10L�ܱպ��ݷ�Ӧ���У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10MPa������÷�Ӧ��ƽ�ⳣ����д��������̣�������������λ��Ч���֣���______��
��T��ʱ��ƽ����A��䵽B��ʱ��ƽ�ⳣ��K��A��______K��B�������������������=������
����֪1g����������ȫ��Ӧ������̬�������ų�����1.536kJ����������Ӧ�ķ�Ӧ�ȡ�H=______��������С�����һλ��
��3��ij����С������ԭ���ԭ������SO2��O2��H2O���Ʊ����ᣬװ����ͼ���缫A��BΪ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ������ʣ�
��B�缫�ĵ缫��ӦΪ______��
����Һ��H+���ƶ���������______���ص�______���أ���A��B����

��1��3FeS2+8O2
6SO2+Fe3O4�У�Fe��SԪ�صĻ��ϼ����ߣ�OԪ�صĻ��ϼ۽��ͣ�3mol FeS2�μӷ�Ӧ����OԪ�صĻ��ϼ۱仯��֪��ת�Ƶĵ���Ϊ8mol��2����2-0��=32mol����1molFeS2�μӷ�Ӧת�Ƶĵ��ӵ����ʵ���Ϊ��
��10.7mol��
�ʴ�Ϊ��10.7mol��
��2������ͼ1��֪����ϵ��ѹǿΪ0.10MPa������SO2��=0.8����
2SO2��g��+O2��g��
2SO3��g��
��ʼŨ�ȣ�0.200.100.0mol?L-1
ת��Ũ�ȣ�0.160.080.16mol?L-1
ƽ��Ũ�ȣ�0.040.020.16mol?L-1
K=
=
=8.0��102��
�ʴ�Ϊ��8.0��102��
��T��ʱ��ƽ����A��䵽B��ʱ���¶Ȳ��䣬���Ի�ѧƽ�ⳣ�����䣬��K��A��=K��B�����ʴ�Ϊ��=
��2mol�������������Ϊ128g����ȫȼ��128g��������������̬��������ų�������Ϊ��1.536kJ/g��128g��196.6kJ�����Է�Ӧ2SO2��g��+O2��g��
2SO3��g���ķ�Ӧ�ȡ�H=-196.6kJ?mol-1��
�ʴ�Ϊ��-196.6kJ?mol-1��
��3���ٸ�ԭ����У�������ʧ���ӱ����������Ը�����Ͷ�ŵ������Ƕ�������������ʧ���Ӻ�ˮ��Ӧ������������Ӻ������ӣ�������Ͷ�ŵ������������������������õ��Ӻ������ӷ�Ӧ����ˮ�����������ˮ�ij��ڷ���֪��B���Ǹ�����A��������������B���ϵĵ缫��ӦʽΪ��SO2-2e-+2H2O�TSO42-+4H+��ԭ��طŵ�ʱ���������ɸ���B��������A��
�ʴ�Ϊ��SO2-2e-+2H2O�TSO42-+4H+��
��ԭ��طŵ�ʱ���������ƶ������������ƶ���������Һ���������ɸ���B��������A��
�ʴ�Ϊ��B��A��
| ||
| 32mol |
| 3 |
�ʴ�Ϊ��10.7mol��
��2������ͼ1��֪����ϵ��ѹǿΪ0.10MPa������SO2��=0.8����
2SO2��g��+O2��g��
| ||
| �� |
��ʼŨ�ȣ�0.200.100.0mol?L-1
ת��Ũ�ȣ�0.160.080.16mol?L-1
ƽ��Ũ�ȣ�0.040.020.16mol?L-1
K=
| c(SO3)2 |
| c(SO2)2?c(O2) |
| 0.162 |
| 0.042��0.02 |
�ʴ�Ϊ��8.0��102��
��T��ʱ��ƽ����A��䵽B��ʱ���¶Ȳ��䣬���Ի�ѧƽ�ⳣ�����䣬��K��A��=K��B�����ʴ�Ϊ��=
��2mol�������������Ϊ128g����ȫȼ��128g��������������̬��������ų�������Ϊ��1.536kJ/g��128g��196.6kJ�����Է�Ӧ2SO2��g��+O2��g��
| ||
�ʴ�Ϊ��-196.6kJ?mol-1��
��3���ٸ�ԭ����У�������ʧ���ӱ����������Ը�����Ͷ�ŵ������Ƕ�������������ʧ���Ӻ�ˮ��Ӧ������������Ӻ������ӣ�������Ͷ�ŵ������������������������õ��Ӻ������ӷ�Ӧ����ˮ�����������ˮ�ij��ڷ���֪��B���Ǹ�����A��������������B���ϵĵ缫��ӦʽΪ��SO2-2e-+2H2O�TSO42-+4H+��ԭ��طŵ�ʱ���������ɸ���B��������A��
�ʴ�Ϊ��SO2-2e-+2H2O�TSO42-+4H+��
��ԭ��طŵ�ʱ���������ƶ������������ƶ���������Һ���������ɸ���B��������A��
�ʴ�Ϊ��B��A��

��ϰ��ϵ�д�
 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
�����Ŀ
 ���H2______0�����������������=��������T��ʱ��1L�ܱ������У�����1molCH4��3molH2O��g����������Ӧ�ڣ�����5min�ﵽƽ�⣬��ʱCH4��ת����Ϊ50%����ӿ�ʼ��ƽ�⣬H2��ƽ����Ӧ����Ϊ______��T��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ______��
���H2______0�����������������=��������T��ʱ��1L�ܱ������У�����1molCH4��3molH2O��g����������Ӧ�ڣ�����5min�ﵽƽ�⣬��ʱCH4��ת����Ϊ50%����ӿ�ʼ��ƽ�⣬H2��ƽ����Ӧ����Ϊ______��T��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ______��