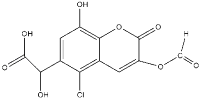
��Ŀ����
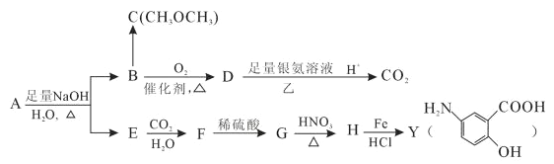
����Ŀ���Ӷ�������ȡ�����л���A���úϺϳɿ��᳦��ҩ��Y��������ѧƷ���ϳ�·����ͼ��
����������Ϣ�ش�
(1)��д��Y�к��������ŵ�����_________________________��
(2)д����Ӧ�۵ķ�Ӧ���ͣ�________________________________��
(3)д����Ӧ�ٵĻ�ѧ����ʽ��_______________________________________��
(4)A��ͬ���칹��I��J����Ҫ��ҽҩ�м��壬��Ũ�����������I��J�ֱ�����![]() ��
��![]() ������I��J���Լ�Ϊ____________________��
������I��J���Լ�Ϊ____________________��
(5)G��ͬ���칹���У�����������������_____________�֡�
���ܷ���������Ӧ �������Ȼ�����Һ������ɫ��Ӧ
���к˴Ź���������ʾ���ֲ�ͬ���͵����շ塣��������֮��Ϊ1:2:2:1�Ľṹ�� ʽΪ____(дһ��)��
(6)A����һ��ͬ���칹��K���ںϳɸ߷��Ӳ���M(![]() )��K ����L(
)��K ����L(![]() )�Ƶá���д����LΪԭ���Ƶ�M �ĺϳ�·������ͼ( ���Լ�����)������ͼʾ�����£�
)�Ƶá���д����LΪԭ���Ƶ�M �ĺϳ�·������ͼ( ���Լ�����)������ͼʾ�����£�![]() ___________________________
___________________________
���𰸡� �Ȼ������ӣ��ǻ� ȡ����Ӧ 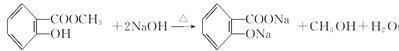 FeCl3��Һ����ˮ 9
FeCl3��Һ����ˮ 9 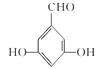 ��
�� ��
��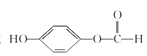 HOOC
HOOC![]() CH2Cl
CH2Cl![]() NaOOC
NaOOC![]() CH2OH
CH2OH![]() HOOC
HOOC![]() CH2OH
CH2OH![]()
![]()
��������������Ҫ�����л���Ľṹ�����ʡ�
(1)Y�к��������ŵ����ƣ��Ȼ����ǻ���
(2)��Ӧ����������Ӧ����Ӧ���ͣ�ȡ����Ӧ��
(3)B�Ǽ״�����Ӧ�ٷ������ڼ��������µ�ˮ�ⷴӦ����Ӧ�Ļ�ѧ����ʽ��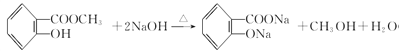 ��
��
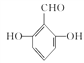
(4)I���д��ǻ���J���з��ǻ������÷��봼���ʵIJ����Լ���I��J�������Լ�ΪFeCl3��Һ����ˮ��
(5)G�����ǻ������ᡣ�ٱ�����G��ͬ���칹�庬��ȩ�����ڱ�����G��ͬ���칹�庬�з��ǻ������ڸ�G��ͬ���칹��������������Ϊ��OH����OH����CHOʱ����6�ֽṹ������������Ϊ��OH����OOCHʱ����3�ֽṹ����9�ֽṹ�����к˴Ź���������ʾ���ֲ�ͬ���͵����շ�����������֮��Ϊ1:2:2:1�Ľṹ��ʽΪ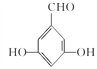 ��
�� ��
��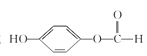 ��
��
(6)��LΪԭ���Ƶ�M�ĺϳ�·������ͼ��HOOC![]() CH2Cl
CH2Cl![]() NaOOC
NaOOC![]() CH2OH
CH2OH![]() HOOC
HOOC![]() CH2OH
CH2OH![]()
![]() ��
��

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
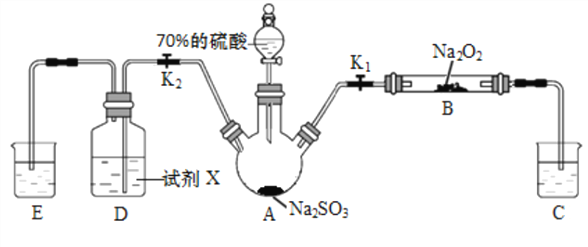
������ҵ��ٳɳ����½������������ϵ�д�����Ŀ����������(POCl2)����Ҫ�Ļ�������ԭ�ϣ��㷺������ҩ��Ⱦ�����ܽ���������ҵ��ij��ȤС��ģ��PCl3ֱ���������Ʊ�POCl3��ʵ��װ��������£�

�й����ʵIJ����������±���
�۵�/�� | �е�/�� | ���� | |
PCl3 | -112 | 75.5 | ��ˮ����H3PO3��HCl����O2����POCl3 |
POCl3 | 2 | 105.3 | ��ˮ����H3PO4��HCl��������PCl3 |
�ش��������⣺
(1)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ___________________________��
(2)Bװ�õ����ó��۲�O2������֮�⡣����__________________________��
(3)Cװ�ÿ��Ʒ�Ӧ��60�桫65����У�����ҪĿ����_______________________��
(4)ͨ��������·����Բⶨ�������ײ�Ʒ��ClԪ�غ�����ʵ�鲽�����£�
��.ȡxg ��Ʒ����ƿ�У���������NaOH ��Һ������ȫ��Ӧ���ϡ���������ԡ�
��.����ƿ�м���0.1000mol/L ��AgNO3��Һ40.00mL��ʹCl-��ȫ������
��.�����м���2mL������������ҡ����ʹ�������汻�л��︲�ǡ�
��.����ָʾ������cmol/LNH4SCN ��Һ�ζ�����Ag+���յ㣬�������� ���VmL��
��֪��Ksp(AgCl)=3.2��10-10��Ksp(AgSCN)=2��10-12
�ٵζ�ѡ�õ�ָʾ����________________(����)��
a.FeCl2 b.NH4Fe(SO4)2 c.���� d.����
��ClԪ�ص������ٷֺ���Ϊ(�г���ʽ)____________________��
�۲���������������Ŀ����_________________�����˲���������ClԪ�غ�������____________�ƫ�� ƫС�����䡱)��