��Ŀ����
����Ŀ���û�ѧ��Ӧԭ���о������仯���������ش���ش���������:
��1����֪: N2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4 kJ/mol
2NH3(g) ��H=-92.4 kJ/mol
N2(g)+O2(g)=2NO(g) ��H=+180.5 kJ/mol
2H2(g)+O2(g)=2H2O(g) ��H=-483.6kJ/mol
����34g��������������ȫ����һ�����������ˮ�������ų�������Ϊ_______��
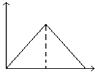
��2��T1�¶�ʱ�����ݻ�Ϊ2 L �ĺ����ܱ������з�����Ӧ: 2NO(g)+O2(g)![]() 2NO2(g) ��H<0��ʵ����:v��=v(NO)����=2v(O2 )����=k��c2(NO)��c(O2)��v��=v(NO2)����=k��c2(NO2) ��k����k��Ϊ���ʳ��������¶�Ӱ�졣�����и���Ӧ�������������ʵ�����ʱ��仯��ͼ��ʾ:
2NO2(g) ��H<0��ʵ����:v��=v(NO)����=2v(O2 )����=k��c2(NO)��c(O2)��v��=v(NO2)����=k��c2(NO2) ��k����k��Ϊ���ʳ��������¶�Ӱ�졣�����и���Ӧ�������������ʵ�����ʱ��仯��ͼ��ʾ:
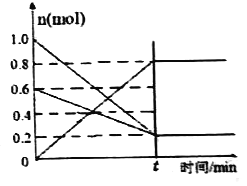
������˵���ܱ����÷�Ӧ�Ѵﵽƽ��״̬����______(�����)��
A.���������ܶȲ��� B.����������ɫ����
C. k����k������ D.2v��(O2)= v��(NO2)
����ѧƽ�ⳣ��K�����ʳ���k����k������ѧ��ϵ��K=_______��
��T1�¶�ʱ����ѧƽ�ⳣ��K=______�����������¶ȸı�ΪT2ʱ����k��=k�� ����T2___T1 (����>������<������=��)��
��3�� �������ǵ�Ԫ����Ҫ�ĺ�����֮һ��25�棬���amol/LHNO2ϡ��Һ��pH=b�����¶���HNO2����ƽ�ⳣ���ľ�ȷ�������ʽΪK=______(�ú�a��b�Ĵ���ʽ��ʾ)����amol/L��NaCN��Һ��0.01mol/L������������Ϻ����ҺpH=7������Һ��![]() =_____(�ú�a �Ĵ���ʽ��ʾ)��
=_____(�ú�a �Ĵ���ʽ��ʾ)��
���𰸡� 452.5 kJ B�� D k ��/k �� 160 �� 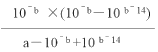 1 /(100a��1)
1 /(100a��1)
�������������������1�����ݸ�˹���ɼ���34g��������������ȫ����һ�����������ˮ�������ų�����������2��������ƽ���־������������ƽ��״̬ʱv��= v���������۸���![]() ������
������![]() �����������¶ȸı�ΪT2ʱ����k��=k������K=1���¶ȸı�ΪT2ʱƽ�ⳣ����С����Ӧ�����ƶ���(3)����K=
�����������¶ȸı�ΪT2ʱ����k��=k������K=1���¶ȸı�ΪT2ʱƽ�ⳣ����С����Ӧ�����ƶ���(3)����K=![]() ���������ݵ���غ㡢�����غ����
���������ݵ���غ㡢�����غ����![]() ��
��
��������1���� N2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4kJ/mol
2NH3(g) ��H=-92.4kJ/mol
�� N2(g)+O2(g)=2NO(g) ��H=+180.5kJ/mol
�� 2H2(g)+O2(g)=2H2O(g) ��H=-483.6kJ/mol
���ݸ�˹�������![]() ����� 2NH3(g)+
����� 2NH3(g)+ ![]() O2(g)= 2NO(g)+ 3H2O(g) ��H= - 452.5 kJ/mol������34g��������������ȫ����һ�����������ˮ�������ų�������452.5 kJ����2������Ӧǰ���������������������������������
O2(g)= 2NO(g)+ 3H2O(g) ��H= - 452.5 kJ/mol������34g��������������ȫ����һ�����������ˮ�������ų�������452.5 kJ����2������Ӧǰ���������������������������������![]() ���ܶ��Ǻ��������������ܶȲ��䲻һ��ƽ������A����������������ɫ������˵��NO2��Ũ�Ȳ��䣬һ���ﵽƽ��״̬����B��ȷ�� C. k����k��ֻ���¶ȱ仯���¶��Ǻ���������k����k���Ǻ�����k����k����������һ��ƽ������C���� 2v��(O2)= v��(NO2)�����淴Ӧ���ʱȵ���ϵ������һ���ﵽƽ��״̬����D��ȷ����ƽ��״̬ʱv��= v������k��c2(NO)��c(O2) =k��c2(NO2)��
���ܶ��Ǻ��������������ܶȲ��䲻һ��ƽ������A����������������ɫ������˵��NO2��Ũ�Ȳ��䣬һ���ﵽƽ��״̬����B��ȷ�� C. k����k��ֻ���¶ȱ仯���¶��Ǻ���������k����k���Ǻ�����k����k����������һ��ƽ������C���� 2v��(O2)= v��(NO2)�����淴Ӧ���ʱȵ���ϵ������һ���ﵽƽ��״̬����D��ȷ����ƽ��״̬ʱv��= v������k��c2(NO)��c(O2) =k��c2(NO2)��![]() ��������ͼ����ƽ��ʱNO��Ũ����0.1mol/L��O2��Ũ����0.1mol/L��NO2��Ũ����0.4mol/L��
��������ͼ����ƽ��ʱNO��Ũ����0.1mol/L��O2��Ũ����0.1mol/L��NO2��Ũ����0.4mol/L�� ![]() ��������Ӧ�������¶���T1�ı�ΪT2��ƽ�ⳣ����С����Ӧ�����ƶ�������T2>T1����(3)25�棬���amol/LHNO2ϡ��Һ��pH=b����
��������Ӧ�������¶���T1�ı�ΪT2��ƽ�ⳣ����С����Ӧ�����ƶ�������T2>T1����(3)25�棬���amol/LHNO2ϡ��Һ��pH=b����![]() �����ݵ���غ���
�����ݵ���غ���![]() �����������غ���
�����������غ���![]() ������K=
������K=![]() =
=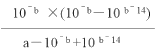 ����amol/L��NaCN��Һ��0.01mol/L������������Ϻ�PH=7����
����amol/L��NaCN��Һ��0.01mol/L������������Ϻ�PH=7����![]() �����������غ�
�����������غ�![]() ��
��![]() ��
��![]() �����ݵ���غ���
�����ݵ���غ���![]() ��
��![]() ��
��![]() ��
��![]() =
=![]() =1 /(100a��1)��
=1 /(100a��1)��

����Ŀ����֪25��ʱ�����ֳ���������ʵĵ���ƽ�ⳣ�����±���ʾ:
����� | H3PO4 | NH3��H2O | C6H5OH |
����ƽ�ⳣ�� | K1=7.5��10-3 K2=7.5��10-8 K3=7.5��10-13 | 1.7��10-5 | 1.1��10-10 |
����˵����ȷ����
A. NaH2PO4��Һ�ʼ���
B. 25��ʱ��0.1moL/L��ˮ��pH=11+lg1.7
C. ����C6H5OH��Na3PO4��Ӧ�����ӷ���ʽΪ:2C6H5OH+PO43-=H2PO4-+2C6H5O-
D. 25��ʱ�����������Ũ�ȵı�����Һ�백ˮ��ϣ������Һ�и�����Ũ�ȹ�ϵ��c(C6H5O-)>c(NH4+)>c(OH-)>c(H+)