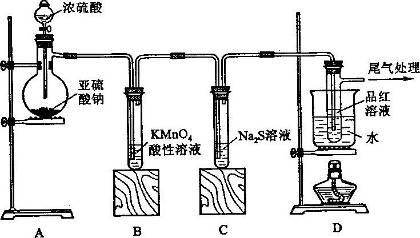
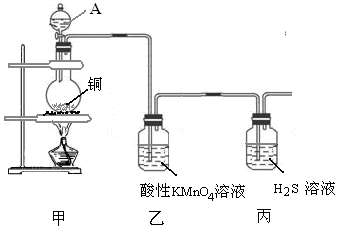
��Ŀ����
ij��ѧ������ȤС��̽��ͭ��Ũ����ķ�Ӧ�����ijЩ��������ʡ�
(��)�ס�����ͬѧ����������ʵ�飺ȡһ������ͭƬ��20 mL 18 mol/L��Ũ�������Բ����ƿ�й��ȣ�ֱ����Ӧ��ϣ��������ƿ�л���ͭƬʣ�࣬ͬʱ������ѧ��֪ʶ��Ϊ���н϶�����ʣ�ࡣ
��1��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ�ǣ�
����֤���������ʵ�鷽����_ (����ĸ)��
a���ټ�������NaNO3����B���ٵ���BaCl2��Һ C���ټ����� D���ٵ���Na2CO3��Һ
��2����ͬѧ���������Ũ�ȵ�ʵ�鷽���Dzⶨ����������������з����в����е��� (����ĸ)��
a�������������建��ͨ��Ԥ�ȳ�����ʢ�м�ʯ�ҵĸ���ܣ���Ӧ�������ٴγ���
b�������������建��ͨ�����Ը��������Һ���ټ�������BaCl2��Һ�����ˡ�ϴ�ӡ������������
c������ˮ���ⶨ�������������(����ɱ�״��)
d�����ű���NaHSO3��Һ�ķ�������������������(����ɱ�״��)
��3����ͬѧ��Ʋⶨ����Ũ�ȵ�ʵ�鷽���ǣ��ⶨ��Ӧ��Ļ��Һ��Cu2���������ڷ�Ӧ�����Һ�м�������Na2S��Һ����ַ�Ӧ���ˡ�ϴ�ӡ������������������ΪW g����÷�Ӧ����Һ�����ΪV mL����ʣ����������ʵ���Ũ��Ϊ mol/L(�ú�W��V�Ĵ���ʽ��ʾ)��
(��)��ͬѧ̽��SO2��BaCl2��Һ�ܷ�Ӧ���ɰ�ɫBaSO3������
��ͬѧ�Ƚ�����������ͨ��ʢ�б���NaHSO3��Һ��ϴ��ƿ���ٻ���ͨ��BaCl2��Һ�У��۲쵽��������ɫ�������ɣ������ɫ���������ֳ���ȫ��������ϡ���ᣬ�ó��������ɱ���SO2���� �ԡ���һ�����ӷ���ʽ�������ɴ˳�����ԭ�� ��
(��) ��1�� Cu��2H2SO4(Ũ)  CuSO4��SO2����2H2O��2�֣� ad ��2�� abc��2�֣���3��
CuSO4��SO2����2H2O��2�֣� ad ��2�� abc��2�֣���3�� 
(��) ��ԭ��2�֣� 2Ba2����2SO2��O2��2H2O=2BaSO4����4H����2�֣�
�������������(��)��1��ͭ��Ũ�����ڼ��������·�Ӧ��������ͭ�����������ˮ����ѧ����ʽΪ��
Cu��2H2SO4(Ũ)  CuSO4��SO2����2H2O����Ӧ��������ͭ���ɣ�֤��������Ӧ������Һ�к��������ӣ�a���������ᣬ�ټ�������NaNO3����ɷ�����Ӧ��3Cu + 2NO3- + 8H+ = 3Cu2+ + 2NO��+ 4H2O��ʣ���ͭ�ܽ⣬���������ɣ����Թܿڱ����ɫ������֤���� b������BaCl2��Һ���������Ӳ���Ӧ������֤���� C���ټ����� ���������Ӳ���Ӧ������֤����D���ٵ���Na2CO3��Һ���������ӷ�Ӧ����ɫ�������ɣ�����֤����ѡad����2����ͬѧ���������Ũ�ȵ�ʵ�鷽���Dzⶨ�������������a�������������к���ˮ����Ҳ�ܱ���ʯ�����գ�������������У�b�����Ը��������Һ���������ữ�ģ�����������ͨ�����Ը��������Һ���ټ�������BaCl2��Һ�����ˡ�ϴ�ӡ����������������������ƫ�����У�c����������������ˮ������ˮ���ⶨ��������������������У�d�������������ڱ���NaHSO3��Һ�����ű���NaHSO3��Һ�ķ�������������������(����ɱ�״��)�����У�ѡabc����3����ͬѧ��Ʋⶨ����Ũ�ȵ�ʵ�鷽���漰��ӦΪ��
CuSO4��SO2����2H2O����Ӧ��������ͭ���ɣ�֤��������Ӧ������Һ�к��������ӣ�a���������ᣬ�ټ�������NaNO3����ɷ�����Ӧ��3Cu + 2NO3- + 8H+ = 3Cu2+ + 2NO��+ 4H2O��ʣ���ͭ�ܽ⣬���������ɣ����Թܿڱ����ɫ������֤���� b������BaCl2��Һ���������Ӳ���Ӧ������֤���� C���ټ����� ���������Ӳ���Ӧ������֤����D���ٵ���Na2CO3��Һ���������ӷ�Ӧ����ɫ�������ɣ�����֤����ѡad����2����ͬѧ���������Ũ�ȵ�ʵ�鷽���Dzⶨ�������������a�������������к���ˮ����Ҳ�ܱ���ʯ�����գ�������������У�b�����Ը��������Һ���������ữ�ģ�����������ͨ�����Ը��������Һ���ټ�������BaCl2��Һ�����ˡ�ϴ�ӡ����������������������ƫ�����У�c����������������ˮ������ˮ���ⶨ��������������������У�d�������������ڱ���NaHSO3��Һ�����ű���NaHSO3��Һ�ķ�������������������(����ɱ�״��)�����У�ѡabc����3����ͬѧ��Ʋⶨ����Ũ�ȵ�ʵ�鷽���漰��ӦΪ��
Cu��2H2SO4(Ũ)  CuSO4��SO2����2H2O��Cu2+ + S2-= CuS��,���ݻ�ѧ����ʽ����ͭ��μӷ�Ӧ������Ĺ�ϵʽ��CuS����2H2SO4����μӷ�Ӧ����������ʵ���ΪW /48mol��ʣ����������ʵ���Ϊ��0.36��W /48��mol�����ʵ���Ũ��Ϊ
CuSO4��SO2����2H2O��Cu2+ + S2-= CuS��,���ݻ�ѧ����ʽ����ͭ��μӷ�Ӧ������Ĺ�ϵʽ��CuS����2H2SO4����μӷ�Ӧ����������ʵ���ΪW /48mol��ʣ����������ʵ���Ϊ��0.36��W /48��mol�����ʵ���Ũ��Ϊ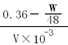 mol/L��
mol/L��
(��) ��ͬѧ�Ƚ�����������ͨ��ʢ�б���NaHSO3��Һ��ϴ��ƿ���ٻ���ͨ��BaCl2��Һ�У��۲쵽��������ɫ�������ɣ������ɫ���������ֳ���ȫ��������ϡ���ᣬ��ó���Ϊ���ᱵ�������ó�����ԭ��Ϊ2Ba2����2SO2��O2��2H2O=2BaSO4����4H����SO2���ֻ�ԭ�ԡ�
���㣺����ʵ�鷽������������ۡ���ѧ����ʽ��д�ͻ�ѧ���㡣

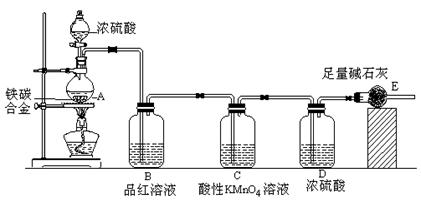
ijѧϰ��ȤС��̽����������ȡʵ��:
(1)��ͬѧ��������ʵ�鷽���Ʊ�����,���к������� (�����,��ͬ)��
| A�����Ȼ�粒�����ȷֽ� |
| B����Ũ��ˮ�����������ƹ����� |
| C�����������ƹ������Ũ��ˮ�� |
| D�����Ȼ��ϡ��Һ�����������ƹ����� |
��ҵ�ϳ�����������ʢװ��Ũ���ᡣij��ȤС���ͬѧ���֣���һ������������Ũ�������ʱ���۲쵽������ȫ�ܽ⣬�������������塣ʵ�������������Լ��� 0.01 mol/L ����KMnO4��Һ��0.10 mol/L KI��Һ��������ˮ��������Һ������ˮ������Э������̽��������Һ������ijɷ֡�
��������롿
��������Һ�еĽ������ӿ��ܺ���Fe2����Fe3���е�һ�ֻ����֣�
�����������п϶����� ���塣
��ʵ��̽����
| | ʵ����� | Ԥ������ | �� �� |
| ��֤����� | ����٣�ȡ����0.01 mol/L ����KMnO4��Һ������������Һ�� | | |
| ����ڣ� | | ����Fe3�� | |
| ��֤����� | ����������ͨ������װ�� | | �������ֻ��������� |
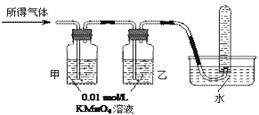
���������ۡ�
��1����ͬѧ�����������ѡ��KSCN��Һ���������KSCN��������ˮ������Һ������ɲ���������̽���������Ƿ���У���˵��ԭ�� ��
��2����ͬѧ������Թ������������H2��Q���壬Ϊ�����������ʵ��װ������ͼ��ͼ�мг�����ʡ�ԣ���
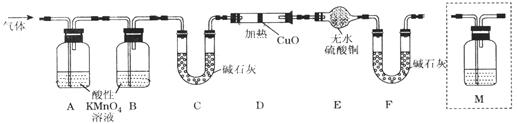
��Q������ԭ���� ���û�ѧ����ʽ��ʾ����
��Ϊȷ��Q�Ĵ��ڣ���M��ʢ�ŵ��Լ�Ϊ ������װ��M������ ��ѡ����ţ���
a��A֮ǰ b��A-B�� c��B-C�� d��C-D��
��װ��ͼ��D��E��F��ϵ������� ��