��Ŀ����
����Ŀ�������Ƕ���˹��ѧ���Ž��з������Ԫ����������150���ꡣ�Ž��з�Ϊ�ü��ֵ�ʱ��δ���ֵ�Ԫ��![]() �����������������������������
�����������������������������![]() �����˿�λ����������ѧ����1875���о���п��(ZnS)ʱ���ֵ��������������Ž��з�Ԥ�Ե���������������Ҳ�Ǻ�Ԥ���о��˵����ơ�
�����˿�λ����������ѧ����1875���о���п��(ZnS)ʱ���ֵ��������������Ž��з�Ԥ�Ե���������������Ҳ�Ǻ�Ԥ���о��˵����ơ�
�ش��������⣺

(1)��д���صĻ�̬ԭ�ӵĵ����Ų�ʽ______���Ž��з�Ԥ�Ե����������������ڵ��֡����������ʹ���ڵ��࣬������Ԫ�ص�ԭ���У�δ�ɶԵĵ�����������______![]() ��Ԫ�ط���
��Ԫ�ط���![]() ��
��
������˵�����п�����ȷ��һ����______��![]() ����ĸ
����ĸ![]()
A.������![]() ʱ����ѹ�ܸ�
ʱ����ѹ�ܸ� ![]() �����������ﲻ�����ڼ�
�����������ﲻ�����ڼ�
C.�����������ˮ��Ӧ ![]() �������������������ķ���
�������������������ķ���
![]() �Ȼ����۵�77.9�棬�����ص��ӻ���ʽ��������������ԭ���ӻ���ʽ��ͬ���Ȼ��ؿռ乹��Ҳ��������ͬ����______��
�Ȼ����۵�77.9�棬�����ص��ӻ���ʽ��������������ԭ���ӻ���ʽ��ͬ���Ȼ��ؿռ乹��Ҳ��������ͬ����______��![]() ����ĸ
����ĸ![]()
A.PCl3B.SO3C.CH3-D.NO3-
(3)�ض����������������ͭ��ȿ��С�
��Cu(OH)2�����ڰ�ˮ���γɵ�������У������ӵĽṹ����ʾ��ͼ��ʾΪ![]() ����
����![]() ����ʾ����λ��
����ʾ����λ��![]() ______��
______��
��п�ĵ�һ������(I1)����ͭ�ĵ�һ�����ܣ���п�ĵڶ�������(I2)ȴС��ͭ�ĵڶ������ܵ���Ҫԭ����______��
(4)2011�꣬�ҹ�������Ϊս�Դ����������ҹ����ش���Լռ���索����80%���ϡ��黯��Ҳ�ǰ뵼����ϣ���ṹ����п���ƣ��侧���ṹ��ͼ��ʾ��
��ԭ����������Ǿ����Ļ���Ҫ��֮һ����ʾ�����ڲ���ԭ�ӵ����λ�á�ͼ��A(0,0,0)��![]() ��
��![]() ����˾����У�����A����Զ�ĺ�����������Ϊ______��
����˾����У�����A����Զ�ĺ�����������Ϊ______��
![]() ������ص�ԭ�Ӱ뾶�ֱ�Ϊacm��bcm���黯�ص�Ħ������Mg/molΪ���ܶ�Ϊ��g/cm3��������ԭ�����ռ�ռ�����ٷ���Ϊw��ԭ������Ŀռ�ռ����
������ص�ԭ�Ӱ뾶�ֱ�Ϊacm��bcm���黯�ص�Ħ������Mg/molΪ���ܶ�Ϊ��g/cm3��������ԭ�����ռ�ռ�����ٷ���Ϊw��ԭ������Ŀռ�ռ����![]() �����ӵ�����Ϊ______mol-1��
�����ӵ�����Ϊ______mol-1��
���𰸡�1s22s22p63s23p63d104s24p1 Ge D BD 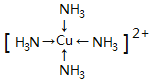 ʧȥ1�����Ӻ�Zn+�۵����Ų�Ϊ3d104s1����Cu+�۵����Ų�Ϊ3d10��
ʧȥ1�����Ӻ�Zn+�۵����Ų�Ϊ3d104s1����Cu+�۵����Ų�Ϊ3d10��![]() �ﵽ���ȶ�״̬����Zn�ĵڶ�������С��Cu�ĵڶ�������
�ﵽ���ȶ�״̬����Zn�ĵڶ�������С��Cu�ĵڶ������� ![]()
![]()
��������
(1)��Ga��31��Ԫ�أ����ڵ�������IIIA�壬����������ԭ����д��������Ų�ʽ���ദ�ڵ������ڵ�IV�塢�ִ��ڵ������ڵ�IIIB�壬���۵����Ų�ʽΪ4s24p2��3d14s2��
��������Al���������ƣ��������Ľ����Ա�Alǿ��ע����������ѹһ��Ƚ�С��Ҳ���¶����{������
(2)GaCl3����ԭ��Gaû�й¶Ե��ӣ��۲���Ӷ���Ϊ3������ԭ���ӻ���ʽ���ռ乹�����Ȼ�����ͬ����Ӧ������������ԭ��Ӧû�й¶Ե��ӡ��۲���Ӷ���Ϊ3��
(3)��������Ϊ[Cu(NH3)4] 2+��Cu2+�ṩ�չ����NH3��Nԭ���ṩ�¶Ե��ӣ��γ���λ����
�ڵ�һ������Cuʧȥ��3d104s1�ĵ��ӣ���Zn��ʧȥ�����ȶ��ṹ3d104s2�ĵ��ӡ�ʧȥ1�����Ӻ�Zn+�۵����Ų�Ϊ3d104s1����![]() �۵����Ų�Ϊ3d10�����ȶ���
�۵����Ų�Ϊ3d10�����ȶ���
(4)�پ����о���A����Զ�ĺ�����A������ߴ��ھ�����Խ����ϣ����ݼ���ԭ�����߾��������Խ��߳��ȵ�![]() ���ú�ɫ����������ƽ������Ϊ�����ⳤ��
���ú�ɫ����������ƽ������Ϊ�����ⳤ��![]() ��
��
���谢��٤������ΪNA/mol����̯�����㾧����Ga��Asԭ����Ŀ���ð���٤��������Ħ��������ʾ���������������㾧����Ga��Asԭ������������ԭ������Ŀռ�ռ���ʼ��㾧������������þ�������![]() �����ܶ�
�����ܶ�![]() ��������з��̼��㡣
��������з��̼��㡣
(1)��Ga��31��Ԫ�أ����ڵ�������IIIA�壬��������Ų�ʽΪ1s22s22p63s23p63d104s24p1��4p�����1������Ϊ�����ӣ��ദ�ڵ������ڵ�IV�塢�ִ��ڵ������ڵ�IIIB�壬���۵����Ų�ʽΪ4s24p2��3d14s2��δ�ɶԵ������ֱ�Ϊ2��1����Ge��δ�ɶԵ�������ࣻ
��A���������ѹһ��Ƚ�С��������1000��ʱ����ѹӦ�ò��Ǻܸߣ�A����
B.���������ڼ������������Ҳ��������ǿ�B����
C.�����Ľ����Ա�Al����ȻAl���ˮ����Ӧ���������������ˮ��Ӧ��C����
![]() ���������Ľṹ���ƣ����������������ķ��࣬D��ȷ��
���������Ľṹ���ƣ����������������ķ��࣬D��ȷ��
�ʺ���ѡ����D��
(2)GaCl3����ԭ��Gaû�й¶Ե��ӣ��۲���Ӷ���Ϊ3������ԭ���ӻ���ʽ���ռ乹�����Ȼ�����ͬ����Ӧ������������ԭ��Ӧû�й¶Ե��ӡ��۲���Ӷ���Ϊ3��
A.PCl3��Pԭ�ӹµ��Ӷ���![]() ���۲���Ӷ���1+3=4��A�����ϣ�
���۲���Ӷ���1+3=4��A�����ϣ�
B.SO3��Sԭ�ӹµ��Ӷ���![]() ���۲���Ӷ���0+3=3��B���ϣ�
���۲���Ӷ���0+3=3��B���ϣ�
C.CH3-��Cԭ�ӹµ��Ӷ���![]() ���۲���Ӷ���1+3=4��C�����ϣ�
���۲���Ӷ���1+3=4��C�����ϣ�
D.NO3-��Nԭ�ӹµ��Ӷ���![]() ���۲���Ӷ���0+3=3��D���ϣ�
���۲���Ӷ���0+3=3��D���ϣ�
�ʺ���ѡ����BD��
(3)��������Ϊ[Cu(NH3)4]2+��![]() �ṩ�չ����NH3��Nԭ���ṩ�¶Ե��ӣ��γ���λ���������ӵĽṹ���Ա�ʾΪ��
�ṩ�չ����NH3��Nԭ���ṩ�¶Ե��ӣ��γ���λ���������ӵĽṹ���Ա�ʾΪ�� ��
��
�ڵ�һ������Cuʧȥ��3d104s1�ĵ��ӣ���Zn��ʧȥ�����ȶ��ṹ3d104s2�ĵ��ӣ���Zn�ĵ�һ�����ܴ���Cu�ĵ�һ�����ܡ�ʧȥ1�����Ӻ�Zn+�۵����Ų�Ϊ3d104s1����Cu+�۵����Ų�Ϊ3d10��Cu+�ﵽ���ȶ�״̬����Zn�ĵڶ�������С��Cu�ĵڶ������ܣ�
(4)�پ����о���A����Զ�ĺ�����A������ߴ��ھ�����Խ����ϣ����ݼ���ԭ�����߾��������Խ��߳��ȵ�![]() ���ú�ɫ����������ƽ������Ϊ�����ⳤ��
���ú�ɫ����������ƽ������Ϊ�����ⳤ��![]() �������������֪�����ⳤΪ1���ʸú�ɫ������ƽ��ľ����Ϊ
�������������֪�����ⳤΪ1���ʸú�ɫ������ƽ��ľ����Ϊ![]() ���ʸú�ɫ����������Ϊ
���ʸú�ɫ����������Ϊ![]() ��
��
���谢��٤������Ϊ![]() ��������Gaԭ����Ŀ
��������Gaԭ����Ŀ![]() ��Asԭ����Ŀ=4�������൱����4����GaAs������������
��Asԭ����Ŀ=4�������൱����4����GaAs������������![]() ��������Ga��Asԭ�������
��������Ga��Asԭ�������![]() ���������Ϊ
���������Ϊ![]() ����
����![]() �����
�����![]() ��
��

����Ŀ�������ͭ�����һ����Ҫ�Ļ���ԭ�ϣ��侧����ɿɱ�ʾΪKxCuy(C2O4)z��wH2O��
(1)ʵ������CuSO4��Һ��NaOH��Һ����Ʊ�Cu(OH)2���ٽ�����Cu(OH)2��KHC2O4��Һ��ϣ����Ʊ������ͭ��ؾ��塣
����֪�����£�Ksp[Cu(OH)2]��2.2��10��20���Ʊ�Cu(OH)2�Ĺ����У�pH��7ʱ����Һ��c(Cu2��)��________��
����֪H2C2O4�Ƕ�Ԫ���ᡣ�����£�Ka1(H2C2O4)��5.4��10��2��Ka2(H2C2O4)��5.4��10��5��KHC2O4ˮ��ƽ�ⳣ������ֵΪ____��
(2)һ�ֲⶨ�����ͭ��ؾ�����ɵķ������£�
�����ȷ��ȡ1.770 0 g��Ʒ����ּ��ȣ�ʣ�����ΪK2CO3��CuO�Ļ�������Ϊ1.090 0 g��
�����ȷ��ȡ1.770 0 g��Ʒ����NH4Cl��Һ�ܽ⡢��ˮϡ�ͣ�������100 mL��
�����ȷ��ȡ�����������Һ25.00 mL����ƿ�У�����ָʾ������Ũ��Ϊ0.050 00 mol��L��1��EDTA����Һ�ζ����յ㡣(��֪Cu2����EDTA��Ӧ�Ļ�ѧ������֮��Ϊ1��1)��
�ظ������ζ��������Σ��й����ݼ�¼���±���
��һ�εζ� | �ڶ��εζ� | �����εζ� | |
����EDTA����Һ �����/mL | 25.92 | 24.99 | 25.01 |
�ٵ�һ�εζ����ĵ�EDTA����Һ���������ƫ���ܵ�ԭ����____(����ĸ)��
A. ��ƿˮϴ��δ����
B. �ζ�ʱ��ƿ����Һ�彦��
C. װEDTA����Һ�ĵζ���ˮϴ��δ��ϴ
D. ��ʼ�ζ�ʱ���ζ��ܼ��첿��δ����Һ��
��ͨ������ȷ������Ʒ�Ļ�ѧʽ(д���������)��______________
����Ŀ���ᴿ��������(������Ϊ����)����ѡ�����Լ�����������ȷ����( )
ѡ�� | ���� | �����Լ� | ���� |
A | �ƾ�(ˮ) | CaO | ���� |
B | ��������(����) | NaOH��Һ | ��Һ |
C | ����(��ϩ) | ���Ը��������Һ | ϴ�� |
D | ��(��) | KI��Һ | ��Һ |
A.AB.BC.CD.D



