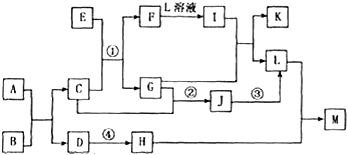
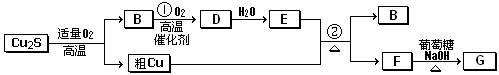
��Ŀ����
����������Li/FeS2��ص������������ʣ�����ˮ�ȷ��ϳɣ�FeSO4��Na2S2O3��S��H2O��200��������Ӧ24Сʱ�����������Ե����ʵ�����Ӧ����������CS2��H2Oϴ�ӡ����P������õ���
��1���ϳ�FeS2���ӷ���ʽΪ ��
��2����ˮϴ��ʱ�����֤��S042-������ ��
��3����֪1.20g FeS2��O2����ȫȼ������Fe2O3��SO2����ų�8.52kJ������FeS2ȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ ��
��4��ȡ�����Ƶõ���������1.1200g���ٶ�ֻ��FeSһ�����ʣ������������������г�ּ��ȣ�����0.8000g����ɫ���壬�������������FeS2������������д��������̣���
��1���ϳ�FeS2���ӷ���ʽΪ
��2����ˮϴ��ʱ�����֤��S042-������
��3����֪1.20g FeS2��O2����ȫȼ������Fe2O3��SO2����ų�8.52kJ������FeS2ȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ
��4��ȡ�����Ƶõ���������1.1200g���ٶ�ֻ��FeSһ�����ʣ������������������г�ּ��ȣ�����0.8000g����ɫ���壬�������������FeS2������������д��������̣���
��������1��FeSO4��Na2S2O3��S��H2O��200��������Ӧ24Сʱ�����������Ե����ʵ�����Ӧ���ɶ�������������Ԫ���غ�͵����غ�д����
��2��֤����������ӳ�����Ҫȡ�����һ��ϴ��Һ��֤��
��3�������Ȼ�ѧ����ʽ��д����д������ע���ʵľۼ�״̬�Ͷ�Ӧ��Ӧ���ʱ䣻
��4��������Ԫ���غ����FeS2������������
��2��֤����������ӳ�����Ҫȡ�����һ��ϴ��Һ��֤��
��3�������Ȼ�ѧ����ʽ��д����д������ע���ʵľۼ�״̬�Ͷ�Ӧ��Ӧ���ʱ䣻
��4��������Ԫ���غ����FeS2������������
����⣺��1��FeSO4��Na2S2O3��S��H2O��200��������Ӧ24Сʱ�����������Ե����ʵ�����Ӧ���ɶ�����������Ӧ�Ļ�ѧ����ʽ��FeSO4+Na2S2O3+S+H2O�TFeS2��+H2SO4+Na2SO4����Ӧ�����ӷ���ʽΪ��Fe2++S2O32-+S+H2O�TFeS2��+2H++SO42-��
�ʴ�Ϊ��Fe2++S2O32-+S+H2O�TFeS2��+2H++SO42-��
��2��֤����������ӳ�����Ҫȡ�����һ��ϴ��Һ��֤��ʵ�鷽��Ϊ��ȡ�������һ��ϴ��Һ���������������ữ������������Һ�м����Ȼ�����Һ���������ֲ��ǻ��DZ�����ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ�������һ��ϴ��Һ���������������ữ������������Һ�м����Ȼ�����Һ���������ֲ��ǻ��DZ�����ϴ�Ӹɾ���
��3����֪1.20g FeS2���ʵ���Ϊ0.01mol����O2����ȫȼ������Fe2O3��SO2����ų�8.52kJ������4mol FeS2ȼ�շ�Ӧ����3408kJ����Ӧ���Ȼ�ѧ����ʽΪ��4FeS2��s��+11O2��g���T2Fe2O3��s��+8SO2��g������H=-3408kJ/mol��
�ʴ�Ϊ��4FeS2��s��+11O2��g���T2Fe2O3��s��+8SO2��g������H=-3408kJ/mol��
��4��ȡ�����Ƶõ���������1.1200g���ٶ�ֻ��FeSһ�����ʣ������������������г�ּ��ȣ�����0.8000g����ɫ����ΪFe2O3��������Ԫ���غ���ʽ��
120g/mol��n��FeS2��+88g/mol��n��FeS��=1.1200g��
n��FeS2��+n��FeS��=2��
������õ���n��FeS2��0.0075mol��
�أ�FeS2��=
��100%=80.36%��
�𣺸�����������FeS2����������Ϊ80.36%��
�ʴ�Ϊ��Fe2++S2O32-+S+H2O�TFeS2��+2H++SO42-��
��2��֤����������ӳ�����Ҫȡ�����һ��ϴ��Һ��֤��ʵ�鷽��Ϊ��ȡ�������һ��ϴ��Һ���������������ữ������������Һ�м����Ȼ�����Һ���������ֲ��ǻ��DZ�����ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ�������һ��ϴ��Һ���������������ữ������������Һ�м����Ȼ�����Һ���������ֲ��ǻ��DZ�����ϴ�Ӹɾ���
��3����֪1.20g FeS2���ʵ���Ϊ0.01mol����O2����ȫȼ������Fe2O3��SO2����ų�8.52kJ������4mol FeS2ȼ�շ�Ӧ����3408kJ����Ӧ���Ȼ�ѧ����ʽΪ��4FeS2��s��+11O2��g���T2Fe2O3��s��+8SO2��g������H=-3408kJ/mol��
�ʴ�Ϊ��4FeS2��s��+11O2��g���T2Fe2O3��s��+8SO2��g������H=-3408kJ/mol��
��4��ȡ�����Ƶõ���������1.1200g���ٶ�ֻ��FeSһ�����ʣ������������������г�ּ��ȣ�����0.8000g����ɫ����ΪFe2O3��������Ԫ���غ���ʽ��
120g/mol��n��FeS2��+88g/mol��n��FeS��=1.1200g��
n��FeS2��+n��FeS��=2��
| 0.8000g |
| 160g/mol |
�أ�FeS2��=
| 0.0075mol��120g/mol |
| 1.1200g |
�𣺸�����������FeS2����������Ϊ80.36%��
���������⿼����������ԭ��Ӧ�Ļ�ѧ����ʽ���Ȼ�ѧ����ʽ��д���������Ӽ�����ƣ�Ԫ���غ�ļ���Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ