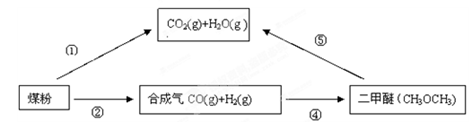
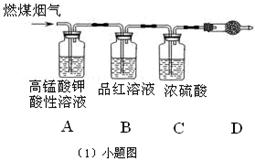
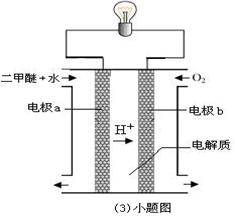
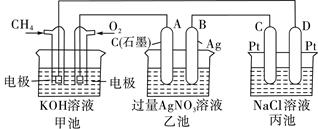
��Ŀ����
������أ�K2FeO4��������һ����Ҫ��������м�ǿ�������ԡ�
��1��������K2FeO4�ܽ���pH=4.74����Һ�У����Ƴ�c(FeO42��)=1.0��10��3mol��L�����������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc(FeO42��)��ʱ��仯�Ľ����ͼ1��ʾ��
��ʵ���Ŀ����_______________________��FeO42��������Ӧ�ġ�H____________0�������������������
��2��������K2FeO4�ֱ��ܽ���pH=4.74��7.00��11.50��ˮ��Һ�У����Ƴ�c(FeO42��)=1.0��10��3 mol��L�������������ã����첻ͬ��ʼpH��ˮ��Һ��K2FeO4ij�����ʵ�Ӱ�죬��仯ͼ���ͼ2��800minʱ����pH=11.50����Һ�У�K2FeO4��Ũ�ȱ���pH=4.74����Һ�иߣ���Ҫԭ����______________��
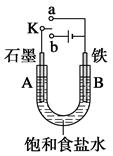
��3����ⷨ�ǹ�ҵ���Ʊ�K2FeO4��һ�ַ���������Ϊ�����������������Һ��Ȼ����������Һ�м���KOH�����ڸ���������Һ�м���KOH�����Ϳ�����������أ�K2FeO4����˵�� �����ʱ����������Ӧ����FeO42�����õ缫��ӦʽΪ______________��
��4����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɸ�����أ�K2FeO4�ڵ�������������ϣ���缫��ӦʽΪFeO42��+3e��+4H2O��Fe(OH)3+5OH������õ���ܷ�Ӧ�����ӷ���ʽΪ_______________��ͼ3Ϊ������ص�غ��ܼ��Ե�طŵ����ߣ��ɴ˿ɵó��ĸ�����ص�ص��ŵ���_______________________��________________________��
��1��̽���¶ȶ�FeO42��Ӱ�죨�¶�Խ�ߣ��������Խ���ȶ�����������2�֣�
��2��pH=11.50ʱ��Һ��OH������Ũ�ȴ�����K2FeO4��ˮ�ķ�Ӧ������С� ��2�֣�
��3��ͬ�¶��£�������ص��ܽ�ȱȸ������Ƶ��ܽ��С ��2�֣�
Fe+8OH����6e��= FeO42��+4H2O ��2�֣�
��4��3Zn+2FeO42��+8H2O=3Zn(OH)2+2Fe(OH)3+4OH�� ��2�֣�
�ŵ�ʱ�䳤��������ѹ�ȶ� ����1�֣�
���������������1����ͼ1���ݿ�֪���¶�Խ�ߣ���ͬʱ����FeO42��Ũ�ȱ仯Խ�죬���������Һƽ��ʱFeO42��Ũ��ԽС���¶�Խ��FeO42��Ũ��ԽС������Ӧ�����ȷ�Ӧ��
�ʴ�Ϊ���¶�Խ�ߣ��������Խ���ȶ������¶�Խ�ߣ����������ˮ��Ӧ������Խ�죩����
��2����ͼ2���ݿ�֪����ҺpHԽС����ͬʱ����FeO42��Ũ�ȱ仯Խ�죬���������Һƽ��ʱFeO42��Ũ��ԽС�����������ˮ�еķ�ӦΪ4FeO42��+10H2O 4Fe(OH)3 +8OH��+3O2����pH=11.50����Һ��OH������Ũ�ȴ�ʹ����ƽ�������ƶ���FeO42��Ũ����������K2FeO4��ˮ�ķ�Ӧ������У�
4Fe(OH)3 +8OH��+3O2����pH=11.50����Һ��OH������Ũ�ȴ�ʹ����ƽ�������ƶ���FeO42��Ũ����������K2FeO4��ˮ�ķ�Ӧ������У�
�ʴ�Ϊ��pH=11.50����Һ��OH������Ũ�ȴ�ʹ����ƽ�������ƶ���������K2FeO4��ˮ�ķ�Ӧ������У�
��3������Ϊ�����������������Һ��Ȼ����������Һ�м���KOH�����ڸ���������Һ�м���KOH�����Ϳ�����������أ�K2FeO4����˵��ͬ�¶��£�������ص��ܽ�ȱȸ������Ƶ��ܽ��С����������ʧ�����ڼ�����Һ�з���������Ӧ����FeO42�����缫��ӦΪ��Fe+8OH��-6e��=FeO42��+4H2O��
�ʴ�Ϊ��ͬ�¶��£�������ص��ܽ�ȱȸ������Ƶ��ܽ��С��Fe+8OH��-6e��=FeO42��+4H2O��
��4��ԭ��صĸ�������������Ӧ�������缫��ӦʽΪ����FeO42��+3e��+4H2O��Fe(OH)3 +5OH���������缫��ӦΪ����Zn-2e��+2OH��=Zn(OH)2�����ݵ缫��Ӧ�ĵ����غ㣬�١�2+�ڡ�3�ϲ��õ���ط�ӦΪ��3Zn+2FeO42��+8H2O=3Zn(OH)2 +2Fe(OH)3 +4OH������ͼ3֪������رȸ��ܼ��Ե�طŵ�ʱ�䳤��������ѹ�ȶ���
�ʴ�Ϊ��3Zn+2FeO42��+8H2O=3Zn(OH)2 +2Fe(OH)3 +4OH�����ŵ�ʱ�䳤��������ѹ�ȶ���
���㣺���ԭ������ѧƽ���Ӱ������

�������������������ʴ��ÿ����ʴ����ʧ�ĸֲ�ռ���������������ķ�֮һ��
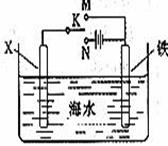
��1��Ϊ�˽���ijˮ�����բ�ű���ʴ�����ʣ����Բ���ͼ����ʾ�ķ��������к�������բ���ϵĹ������R���Բ���________(����ĸ)��
| A��ͭ | B���� |
| C��п | D��ʯī |

 ������ȫ������pH��3.2ʱ
������ȫ������pH��3.2ʱ
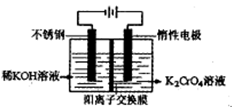
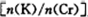 Ϊd�����ʱ�ĸ���ص�ת����Ϊ ��
Ϊd�����ʱ�ĸ���ص�ת����Ϊ ��
 R2Cu���л��ࣩ+ 2H����ˮ�ࣩ
R2Cu���л��ࣩ+ 2H����ˮ�ࣩ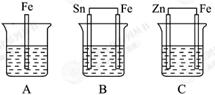
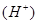 ���� �������С�����䡱����
���� �������С�����䡱����