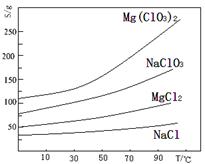
��Ŀ����
ijͭ��ʯ��Ҫ��Cu2(OH)2CO3����������Fe��Si�Ļ����ʵ�����Դ�ͭ��ʯΪԭ���Ʊ�CuSO4��5H2O��CaCO3�����ֲ������£�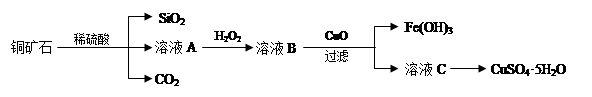
��ش��������⣺
��1����ҺA������Cu2+�⣬�����ܺ��еĽ���������________(�����ӷ���)������ҺA�м���H2O2��Ӧ�����ӷ���ʽ��______________��
��2���������ɵ�CO2��ȡ����̼��ơ��Ʊ�ʱ�������Ȼ�����Һ��ͨ�백������ͨ��CO2��
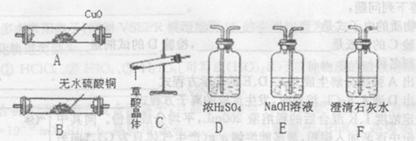
��ʵ����ͨ�����ü����Ȼ�狀��������ƻ����ķ�����ȡ������ijѧϰС��ѡȡ��ͼ��������װ����ȡ���ռ������İ�����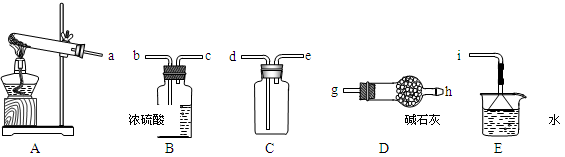
����������������Ӹ������ӿڣ�����Ϊ��ȷ��˳��Ϊa��______��______��______��______�� i��������i����©����������______________��
��ʵ�����л����ù����������ƺ�Ũ��ˮ��ȡ�����������������ʺ���ɸ�ʵ��ļ���װ����
_________(����)
��3���ⶨͭ��ʯ��Cu2(OH)2CO3�����ٷֺ����ķ����ǣ�a����1.25gͭ��ʯ��ȡ��CuSO4��5H2O����ƿ�У���������ˮ��ȫ�ܽ⣻b������Һ�м���100mL0.25mol/L������������ҺʹCu2+��ȫ������c�����ˣ�d����Һ�е�����������Һ��0.5mol/L����ζ����յ㣬����10mL���ᡣ��ͭ��ʯ��Cu2(OH)2CO3��������Ϊ_____________��
��1��Fe2+��Fe3+��2�֣���2Fe2++H2O2 +2H+��2Fe3++2H2O ��2�֣�
��2����a��g��h��e��d�� i ��2�֣�����ֹ������1�֣�����A��2�֣� ��3��88.8%��2�֣�
���������������1��Cu2(OH)2CO3�Լ�Fe��Si�Ļ�������ϡ���ᷴӦ��������ͭ������������������������������ϡ�����Ӧ��������Һ��A�г�����Cu2+�⣬�����ܺ��еĽ���������Fe2+��Fe3+��˫��ˮ����ǿ�����ԣ��ܰ����������������������ӣ���������ҺA�м���H2O2��Ӧ�����ӷ���ʽ��2Fe2++H2O2 +2H+��2Fe3++2H2O��
��2���ٸ���װ��ͼ��֪��Aװ�����Ʊ������ġ��������ɵİ����к���ˮ������������Ҫ���ѡ�ü�ʯ�Ҹ���������ܶ�С�ڿ����ģ��Ұ�����������ˮ������Ӧ���������ſ������ռ�������Ҫ������İ����������գ������ȷ�IJ���˳����a��g��h��e��d�� i��������������ˮ�������i������©���������Ƿ�ֹ������
���ù����������ƺ�Ũ��ˮ��ȡ���������������Ҫ��Һ©������Ӧ����Ҫ���ȣ��Ұ����������ſ������ռ���������ȷ�Ĵ�ѡA��
��3��������������ʵ�����0.01L��0.5mol/L��0.005mol������ݷ���ʽNaOH��HCl��NaCl��H2O��֪�������ᷴӦ������������0.005mol���������Ƶ����ʵ�����0.1L��0.25mol/L��0.025mol����������ͭ��Ӧ������������0.025mol��0.005mol��0.020mol������ݷ���ʽ2NaOH��CuSO4��Cu(OH)2����Na2SO4��֪������ͭ�����ʵ�����0.020mol��2��0.010mol�����Ը���ԭ���غ��֪��ͭ��ʯ��Cu2(OH)2CO3�����ʵ�����0.010mol��2��0.005mol������Cu2(OH)2CO3��������Ϊ ��100%��88.8%��
��100%��88.8%��
���㣺����������ԭ����ʽ��ƽ�������Ʊ������ʺ����IJⶨ�Լ�ʵ�鷽������������۵�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�(4��)�����й�ʵ��������жϲ���ȷ���� _____________������ţ���ѡ�۷֣���
| A������һ�����ʵ���Ũ����Һ������ʱ���ӿ̶��ᵼ��������ҺŨ��ƫ�� |
| B������Ũ����մ��Ƥ���ϣ�����������������Һ��ϴ |
| C������ϡ����ʱ���������ձ��м���һ�����������ˮ���ٱ�������Ũ����߽��� |
| D��100 mL����ƿ����������95 mL 0.1 mol/L NaCl��Һ |
F���۲��Ԫ����ɫ��Ӧ�IJ����ǣ��Ƚ���˿����ϡ������ϴ�ӣ�Ȼ��պȡ�����Ȼ��أ����ھƾ��ƵĻ����Ͻ������գ�����ɫ�ܲ������й۲졣
G������CCl4���ƾ���������ȡ��ˮ�е��塣
ijͬѧ��������ͭ����ᾧˮ�����IJⶨʵ�顣���������գ�
��ʵ�鲽�衿
��1����_______�����������ƣ���ͬ��ȷ������������������
��2���ڴ������м���Լ2 g��ϸ������ͭ���壬��������
��3����ʢ������ͭ����Ĵ������������������������ȣ�ֱ����ɫ��ȫ��ף�Ȼ�����������____________����ȴ�����£���������
��4���ظ�(3)��ʵ����к��ز�����ֱ�����γ������������0.001 g��
�����ݼ�¼�봦����
| | ��һ��ʵ�� | �ڶ���ʵ�� |
| ������������g�� | 29.563 | 30.064 |
| ������������������g�� | 31.676 | 32.051 |
| ���غ�����������ͭ��������g�� | 30.911 | 31.324 |
| x��ֵ | 5.05 | 5.13 |
�����ϱ��е����ݴ�����������㱾��ʵ���������Ϊ______%����֪x������ֵΪ5����
�����������ۡ�
��1����һ��ʵ�飬������Ҫ����________�Σ������֣���ͬ����������Ҫ����_________�Ρ�
��2�����ز�����Ŀ����__________________��
��3���ظ�����ʵ����xƽ��ֵ��Ŀ����_____________________________��
��4��ʵ��ֵ������ֵƫ���ԭ�������________�����ţ���
a�����ȹ������о��彦�� b��������Ʒ�к��м��Ȳ��ӷ�������
c��ʵ��ǰ��������泱ʪ d���������պ�ֱ�ӷ��ڿ�������ȴ
�������岻��ȱ�ٵ���Ԫ�أ����뺬���Ļ�����ɲ��������������ơ����г���һ�ֳ����IJ���ҩƷ���±���˵����IJ������ݡ�
[���]ÿƬ������������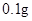 [��Ӧ֢]����ȱ����ƶѪ֢��Ԥ���������á� [�����÷�]����Ԥ���� 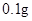 /�գ����������� /�գ����������� �� �� /�ա� /�ա�С������Ԥ���� 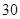 �� �� /�գ������� /�գ������� �� ��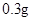 /�� /��[����]�ܹ⡢�ܷ⡢�ڸ��ﴦ���档 |
��1����ҩƷ��Fe2+�Ỻ�����������ҹ涨��ҩ����Fe2+�������ʣ��Ѿ�������Fe2+��������Fe2+�������ı�ֵ������10.00% �������ٷ��á�
��ʵ���ҿɲ���H2SO4�ữ��KMnO4��Һ���ԡ������ơ��е�Fe2+���еζ�(����ҩƷ�������ɷݲ���KMnO4��Ӧ)����д���÷�Ӧ�����ӷ���ʽ�� ��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ�IJ������������������ձ�����ͷ�ι��⣬���� ��
��ijͬѧ��������еζ���ʽ���гֲ�����ȥ������������� ��������ĸ��ţ�

��2��������������Ԫ����������Ϊ20.00%�ġ������ơ�10.00 g������ȫ������ϡH2SO4�У����Ƴ�1000 ml��Һ��ȡ��20.00 ml����0.01000 mol?L-1��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00 ml ����ҩƷ��Fe2+��������Ϊ ��
��3����֪������Ϊ��Ԫ�л����ᣬ��23.6 g���������Һ��4.0 mol?L-1 100.0 ml������������Һǡ����ȫ�к͡��˴Ź�����������ʾ�������������ͼ��ֻ���������շ塣д����������Һ������������Һ��ȫ�к͵Ļ�ѧ����ʽ(�л���д�ṹ��ʽ) ��
����ʯ����Ҫ�ɷ�ΪCaCO3��MgCO3������������Fe��Si�Ļ����ʵ��������ʯΪԭ����Mg(OH)2��CaCO3���������£�
ʵ���������Ҫ�����ݼ��±���
| | �������↑ʼ����ʱ��pH | �������������ȫʱ��pH |
| Fe3+ | 1.9 | 3.2 |
| Fe2+ | 7.0 | 9.0 |
| Mg2+ | 9.5 | 11.0 |
��ش��������⣺
(1)����������IJ��������� ����ҺA�к���Ca2��,Mg2��,Fe2��,Fe3�������Լ��ٿ�ѡ��
(����ĸ��
A.KMnO4 B.Cl2 C.H2O2
(2)��Ҫ���÷�Ӧ���������ɵ�CO2������ҺD����ȡCaCO3����Ҫ��ͨ���һ�������� ��Ȼ��ͨ��CO2��ͨ��CO2����ʱ����ұߵ�װ��ͼ����������������������Һ���������������ӵ�������ѡ����
(3)���ݱ����ṩ�������жϣ�Fe(OH)3��Fe(OH)2��Mg(OH)2���ܶȻ�������С���������˳��Ϊ ��
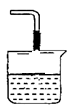
(4)������ȡ��Mg(OH)2�����뵽ij����ϩ��֬�У���֬��ȼ�Դ�ͣ�Mg(OH)����ȼ���õ���Ҫԭ���� ��
���з����п����������к�ˮ��������
| A�����˷� | B����ȡ�� | C����Һ�� | D������ |