��Ŀ����
��ͼ��ʾΪ���������Ʊ������롢�����������֤�IJ�������װ��(�����豸���г̶ֹ�װ�þ���ȥ)�������Ҫ��������и���(����װ�ÿ�����ѡ�ã���Ҫʱ���ظ�ѡ��)��
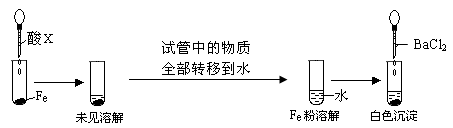
(1)���������ͨ��CO��CO2�Ļ�����壬E�ڷ���CuO��ѡ��װ�û�ô��������CO������֤�仹ԭ�Լ����������ѡװ�õ�����˳��Ϊ________(����ĸ)������֤CO���������������________��
(2)ֹͣCO��CO2��������ͨ�룬E�ڷ���Na2O2����A�D��E�D��D�D��B�D��Hװ��˳����ȡ���������O2������O2�����Ҵ�����ʱ������aӦ________������bӦ________����Ҫ���ȵ�����װ����________(�����)��m�з�Ӧ�Ļ�ѧ����ʽΪ________��
(1) ACBECF��A��B֮���Cװ������Һ���ֳ��壬E��F֮���Cװ������Һ����� (2)�رգ���k��m��2CH3CH2OH��O2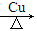 2CH3CHO��2H2O
2CH3CHO��2H2O
����

��ϰ��ϵ�д�
�����Ŀ
�±���ʵ�����Ʊ�������й����ݣ�
| ��� | ʵ������ | ʵ��ԭ�� | ���巢��װ�� |
| �� | ������ | H2O2�D��O2 | |
| �� | �ư��� | NH4Cl�D��NH3 | |
| �� | ������ | HCl�D��Cl2 | |
(1)�ϱ������У����Ʊ����̿�������ѡ����ʵ�����������ʵ�ֵ���________(������Ļ�ѧʽ����ͬ)���ӷ�Ӧԭ���������Բ�ͬ�����������������________��
(2)���ݱ�������ʵ��ԭ����������װ����ѡ����ʵ����巢��װ�ã������������ϱ��еĿո��С�

(3)���������Ʊ�O2��װ���Ʊ�NH3����ѡ����Լ�Ϊ________________��
(4)�Ʊ�Cl2����8 mol��L��1������100 mL������12 mol��L��1�����������ơ�
��Ϊ������Ƶľ�ȷ�ȣ���ȡŨ�����������ѡ�������е�________(�����)��

�����ƹ����У�����ͼ���������⣬����Ҫ��������________��________��________��
I������������ȷ����____________��
| A����Ũ���ᱣ������ɫ����ƿ�� |
| B��������ȡ�����ײ�����ˮ���и� |
| C�����������������IJ�����������Ͱ |
| D��������ԭ����ͭʵ���ȼ�����ͨ���� |
F����������ˮ������ɫ����ƿ��
G����ʪ��ĵ��۵⻯����ֽ����NO2��������
II������ʵ������ȡ�����ķ�Ӧԭ����ʵ��װ�ã��ش��������⣺
��1��д������NH4Cl��Ca(OH)2��ȡNH3�ķ�Ӧ����ʽ��_______________________________
��2������������ֻ������ˮ���ռ�����__________(��ѡ����)��
a��NH3��������b��NO��������c��NO2 d��H2 e��CO2
��3��Ϊ�β��ü���NH4Cl����ķ�����ȡ������_____________________________________
��4����μ��鰱�����ռ����Թܣ�_______________________________________________[

 LaNi5H6(s)��H����31.77 kJ��mol��1��
LaNi5H6(s)��H����31.77 kJ��mol��1�� Si3N4��6CO��Si3N4����________���壬��������Ӧ��������Ϊ________��
Si3N4��6CO��Si3N4����________���壬��������Ӧ��������Ϊ________��
 5NaCl��NaClO3��3H2O�����Ʋ��ڼ���NaClO��Һʱ������Ӧ�Ļ�ѧ����ʽ��______________________________��
5NaCl��NaClO3��3H2O�����Ʋ��ڼ���NaClO��Һʱ������Ӧ�Ļ�ѧ����ʽ��______________________________��
 8SO2+2Fe2O3,�÷�Ӧ�б�������Ԫ������������(��Ԫ�ط���)�����÷�Ӧת��2.75 mol����ʱ,���ɵĶ��������ڱ�״���µ����Ϊ��������L��
8SO2+2Fe2O3,�÷�Ӧ�б�������Ԫ������������(��Ԫ�ط���)�����÷�Ӧת��2.75 mol����ʱ,���ɵĶ��������ڱ�״���µ����Ϊ��������L�� 



