��Ŀ����
��֪һ���¶Ⱥ�ѹǿ�£��ϳɰ���Ӧ��N2��g��+3H2��g�� 2NH3��g����H=-92��0KJ��mol-1����1mol N2��3mol H2����һ�ܱ������У����ֺ��º�ѹ���ڴ�������ʱ���з�Ӧ���ﵽƽ��ʱ�����N2��ת����Ϊ20%��������ͬ�����£���ʼʱ�ڸ������г���2mol NH3����Ӧ�ﵽƽ��ʱ�������仯��
2NH3��g����H=-92��0KJ��mol-1����1mol N2��3mol H2����һ�ܱ������У����ֺ��º�ѹ���ڴ�������ʱ���з�Ӧ���ﵽƽ��ʱ�����N2��ת����Ϊ20%��������ͬ�����£���ʼʱ�ڸ������г���2mol NH3����Ӧ�ﵽƽ��ʱ�������仯��
 2NH3��g����H=-92��0KJ��mol-1����1mol N2��3mol H2����һ�ܱ������У����ֺ��º�ѹ���ڴ�������ʱ���з�Ӧ���ﵽƽ��ʱ�����N2��ת����Ϊ20%��������ͬ�����£���ʼʱ�ڸ������г���2mol NH3����Ӧ�ﵽƽ��ʱ�������仯��
2NH3��g����H=-92��0KJ��mol-1����1mol N2��3mol H2����һ�ܱ������У����ֺ��º�ѹ���ڴ�������ʱ���з�Ӧ���ﵽƽ��ʱ�����N2��ת����Ϊ20%��������ͬ�����£���ʼʱ�ڸ������г���2mol NH3����Ӧ�ﵽƽ��ʱ�������仯��| A������18��4KJ���� | B���ų�73��6KJ���� |
| C���ų�18��4KJ���� | D������73��6KJ���� |
D
�����������ʼʱ�ڸ������г���2mol NH3����ʼʱ�ڸ������г���1mol N2��3mol H2��ȣ�ƽ���ǵ�Ч�ģ����Բ��ǰ����ķֽ�����80��������Ӧ������1.6mol������������ȵ�������92��0KJ��mol-1��2��1.6mol��73��6KJ����ѡD��
�������������Ӧ�Ƿ��ȷ�Ӧ�����淴Ӧ��һ�������ȷ�Ӧ�����Ȿ��Ĺؼ�����Ҫȷ�жϳ�����ƽ���ǵ�Ч�ġ�

��ϰ��ϵ�д�
�����Ŀ
 2NH3�����ֱ���t��ʱ�ⶨ����NH3�������������ͼ���ң�
2NH3�����ֱ���t��ʱ�ⶨ����NH3�������������ͼ���ң�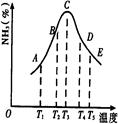
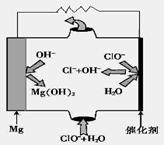
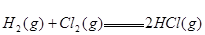 ��
��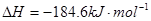 ����Ӧ
����Ӧ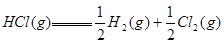 �ġ�HΪ���� ��
�ġ�HΪ���� ��
 O2(g)===H2O(1) ��H=��285.6kJ/mol
O2(g)===H2O(1) ��H=��285.6kJ/mol