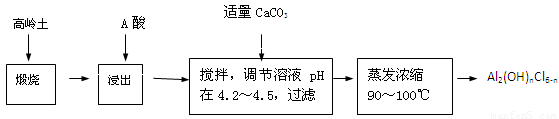
��Ŀ����
��12�֣���֪pHΪ4��5�������£�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⡣ijѧ���õ�ⴿ����CuSO4��Һ�ķ����������ݵ缫������Cu��������n���Լ��缫�ϲ�������������V mL ��״�������ⶨCu�����ԭ���������������£�
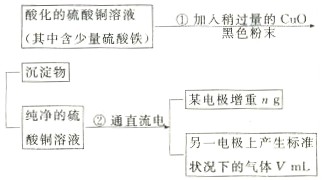
�ش��������⣺
��1������CuO�������� ��
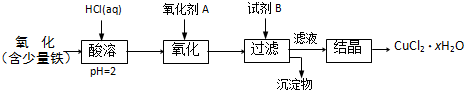
��2������������õIJ�����������ͼ��ʾ����A��B�ֱ���ֱ����Դ�� �� �����������������
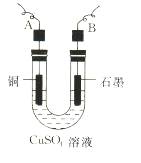
��3����ʼ����U�ι��п��Թ۲쵽�������У� ���������ӷ���ʽΪ ��
��4������ʵ������б�Ҫ���� ����д��ĸ����
��A���������ǰ�ĵ缫����������B�����缫�ں�ɳ���ǰ������������ˮ��ϴ����C�����µ���缫��������ͭ������ϴ����������D�������ɳ��صIJ����б��밴����ɡ��������ٺ�ɡ��ٳ��������У���E�����п������ڵ�����£���ɵ缫�����õ��º�ɵķ�����
��5��ͭ�����ԭ������Ϊ ���ô���m��V�ļ���ʽ��ʾ����
��1��ͨ������H+��������Һ��pHʹ֮���ߣ���Ŀ����ʹFe3+���ˮ���γ�Fe(OH)3��������ȥ��2�֣���2������������1�֣�
��3��ʯī���������ݳ�����Һ��ɫ��dz��2�֣� 2Cu2++2H2O 2Cu��+O2��+4H+ ��2�֣���4��A��B��D��E��2�֣���5��11200n/ V ��2�֣�
2Cu��+O2��+4H+ ��2�֣���4��A��B��D��E��2�֣���5��11200n/ V ��2�֣�
����������1����Ϊ��pHΪ4��5�������£�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣬����������������������Ҫ��ȥ����ͭ��Һ�е�Fe3+���ͱ���������Һ��pHֵ��ͬʱ�����������µ����ʣ���˿�����������ͭ�к������ﵽĿ�ġ���2����Ϊ��Ӧ�б���������壬����ͭ�缫������������ֻ��������������A�Ǹ�����B����������3��ʯī��������Һ�е�OH���ŵ����������ͭ����������Һ�е�Cu2���ŵ�����ͭ������Һ��Cu2����Ũ�ȼ�С����Һ��ɫ��dz���������ӷ���ʽΪ2Cu2++2H2O 2Cu��+O2��+4H+ ����4��Ҫ����������ͭ���������ͱ���������ǰ��ĵ缫���������ڵ缫�����������Һ�����ӣ���˱���������ˮ��ϴ��Ϊ��ֹ�缫�����ˮ�ֲ���������Ҫ���2�Ρ�ͭ�ڼ��ȵ�����»�����������������ԭ��Ӧ��������п������ڵ�����£���ɵ缫�����õ��º�ɵķ��������µ���缫��������ͭ���ڲ����ϲ���ʵ�ģ�Ҳ���ܿ��ξ�����5�����������ΪVml���������ݵ������ӷ���ʽ��2Cu2++2H2O
2Cu��+O2��+4H+ ����4��Ҫ����������ͭ���������ͱ���������ǰ��ĵ缫���������ڵ缫�����������Һ�����ӣ���˱���������ˮ��ϴ��Ϊ��ֹ�缫�����ˮ�ֲ���������Ҫ���2�Ρ�ͭ�ڼ��ȵ�����»�����������������ԭ��Ӧ��������п������ڵ�����£���ɵ缫�����õ��º�ɵķ��������µ���缫��������ͭ���ڲ����ϲ���ʵ�ģ�Ҳ���ܿ��ξ�����5�����������ΪVml���������ݵ������ӷ���ʽ��2Cu2++2H2O 2Cu��+O2��+4H+ �ɼ���ͭ�����ԭ��������
2Cu��+O2��+4H+ �ɼ���ͭ�����ԭ��������

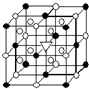 A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������