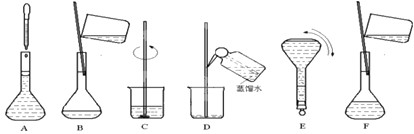
��Ŀ����
��1��NaCl����500mL4.00mol?L-1NaCl��Һ������������ҩ�ס����������_______�����������ƣ���
��2����������Ҫ��ȡ______gNaCl��
��3���ڳ�ȡNaCl���������в�����
�ٵ��ܽ��NaCl���¶�������һ�º��ز�����ע��500mL����ƿ�У�
��������ƿ��С�ļ�����ˮ��Һ��ӽ����α���2��3cm�������ý�ͷ�ιܼ�����ˮ��ʹ��Һ�İ���ײ���ƿ���Ļ��α������У�
����ʢNaCl���ձ���ע������ˮԼ100ml�����ò�����������ʹ���Ͼ��ȣ�
��������ˮϴ���ձ��Ͳ�����2��3�Σ�����ϴ��Һȫ��ע������ƿ��
���������У���ȷ��˳���ǣ�����ţ�______��
��4����ȡNaClʱ���뱻�������̣�10g���������룩�������Ƶ�NaCl��ҺŨ����______�����ƫ�ߡ�����ƫ�͡�������Ӱ�족����ͬ��������������Һʱ����ƿ������ˮϴ����û��������Ƶ�NaCl��ҺŨ����______����δ������ˮϴ���ձ��ڱڻ�δ��ϴ��Һע������ƿ�������Ƶ�NaCl��ҺŨ����______��������ʱ���ӿ̶��ߣ������Ƶ�NaCl��ҺŨ����______��
��5���������Ӧ������ƿ�е�NaCl��Һת�Ƶ�����ྻ��______�д�ţ��Ǻ����Ӳ����ϱ�ǩ��
�⣺��1������500mL4.00mol?L-1NaCl��Һ�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬�ò��������裬�����ܽ⣬�ָ����º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
������Ҫ��������������ƽ���ձ�����������500ml������ƿ����ͷ�ιܡ�ҩ�ף�
ʹ�û���Ҫ������ƽ���ձ���500ml������ƿ����ͷ�ιܣ�
�ʴ�Ϊ��������ƽ���ձ���500ml������ƿ����ͷ�ιܣ�
��2�����Ȼ��Ƶ�����Ϊm=0.5L��4.00mol?L-1��58.5g/mol=117.0g�����������и�ʴ�ԣ��׳��⣬Ӧ����С�ձ���Ѹ�ٳ������ʴ�Ϊ��2.0��
��3���ɣ�1���в��������֪����ȷ��˳���Ǣۢ٢ܢڣ��ʴ�Ϊ���ۢ٢ܢڣ�
��4�������Ȼ��ƹ���ʱ����������ʵ�λ�öԵ�����δʹ�����룬���Ȼ��Ƶ�������Ӱ�죬��������ҺŨ����Ӱ�죻
��ʹ�����룬�Ȼ��Ƶ�ʵ��������С��������Һ��Ũ��ƫ�ͣ�����ʹ�����룬������ҺŨ��ƫ�ͣ�
�����Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
�ձ�δ����ϴ�ӣ������Ȼ���մ���ձ����벣�����ϣ�ϴ��Һ�к��������Ȼ��ƣ�����������ƿ���Ȼ��Ƶ�ʵ�����ʵ�����С����ҺŨ��ƫ�ͣ�
����ʱ����������ƿ�̶��ߣ�ʹ��Һ�����ƫ�ͣ�������ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ͣ���Ӱ�죻ƫ�ͣ�ƫ�ߣ�
��5������ƿ���ܳ�ʱ��ʢ����Һ����õ���ҺӦ�����Լ�ƿ�в����ñ�ǩ���棬�ʴ�Ϊ���Լ�ƿ��
��������1������������Һ�IJ������ѡ������������
��2������n=cv������Ȼ��Ƶ����ʵ������ٸ���m=nM���������Ȼ��Ƶ�������
��3������������Һ�IJ���������в���˳�������
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=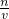 �����жϣ�
�����жϣ�
��5������ƿ���ܳ�ʱ��ʢ����Һ����õ���ҺӦ�����Լ�ƿ�в����ñ�ǩ���森
���������⿼����Һ�����ƣ��ѶȲ��ؼ������Һ���Ƶ�ԭ����ͨ��c=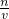 ���Լ������⣮
���Լ������⣮
������Ҫ��������������ƽ���ձ�����������500ml������ƿ����ͷ�ιܡ�ҩ�ף�
ʹ�û���Ҫ������ƽ���ձ���500ml������ƿ����ͷ�ιܣ�
�ʴ�Ϊ��������ƽ���ձ���500ml������ƿ����ͷ�ιܣ�
��2�����Ȼ��Ƶ�����Ϊm=0.5L��4.00mol?L-1��58.5g/mol=117.0g�����������и�ʴ�ԣ��׳��⣬Ӧ����С�ձ���Ѹ�ٳ������ʴ�Ϊ��2.0��
��3���ɣ�1���в��������֪����ȷ��˳���Ǣۢ٢ܢڣ��ʴ�Ϊ���ۢ٢ܢڣ�
��4�������Ȼ��ƹ���ʱ����������ʵ�λ�öԵ�����δʹ�����룬���Ȼ��Ƶ�������Ӱ�죬��������ҺŨ����Ӱ�죻
��ʹ�����룬�Ȼ��Ƶ�ʵ��������С��������Һ��Ũ��ƫ�ͣ�����ʹ�����룬������ҺŨ��ƫ�ͣ�
�����Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
�ձ�δ����ϴ�ӣ������Ȼ���մ���ձ����벣�����ϣ�ϴ��Һ�к��������Ȼ��ƣ�����������ƿ���Ȼ��Ƶ�ʵ�����ʵ�����С����ҺŨ��ƫ�ͣ�
����ʱ����������ƿ�̶��ߣ�ʹ��Һ�����ƫ�ͣ�������ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ͣ���Ӱ�죻ƫ�ͣ�ƫ�ߣ�
��5������ƿ���ܳ�ʱ��ʢ����Һ����õ���ҺӦ�����Լ�ƿ�в����ñ�ǩ���棬�ʴ�Ϊ���Լ�ƿ��
��������1������������Һ�IJ������ѡ������������
��2������n=cv������Ȼ��Ƶ����ʵ������ٸ���m=nM���������Ȼ��Ƶ�������
��3������������Һ�IJ���������в���˳�������
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
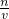 �����жϣ�
�����жϣ���5������ƿ���ܳ�ʱ��ʢ����Һ����õ���ҺӦ�����Լ�ƿ�в����ñ�ǩ���森
���������⿼����Һ�����ƣ��ѶȲ��ؼ������Һ���Ƶ�ԭ����ͨ��c=
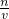 ���Լ������⣮
���Լ������⣮ 
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ