��Ŀ����
3��Ag/��-Al2O3��ʯ�ͻ�ѧ��ҵ��һ����Ҫ����������Ag������ã���-Al2O3�������Ҳ��������ᣬ�ô����Ļ���ʵ����ͼ1��ʾ�����е�ת����ӦΪ��6AgCl+Fe2O3��3Ag2O+2FeCl3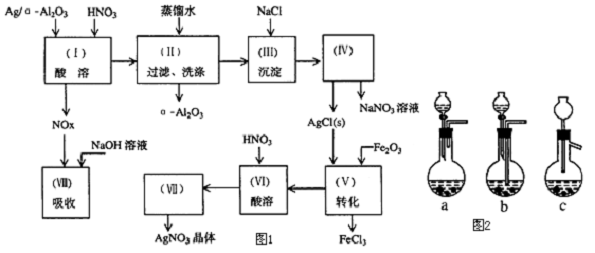
�Ķ�����ʵ�����̣����������գ�
��1��Ag/��-Al2O3�����ܽ�Ӧ��ѡ��װ�ã�ͼ2��a��ѡ��a��b��c����
��2����ʵ������������������ˮ��������ˮ����ϴ�ӣ����ᷢ����ѧ��Ӧ�����ӷ���ʽAg++Cl-=AgCl����
��3��ʵ��������������貣������Ϊ©�����ձ�������������д���֣���
��4��ʵ�������������AgNO3��Һ���AgNO3������Ҫ���е�ʵ���������Ϊ��bd����ѡ�۷֣���
��a������ ��b������ ��c������ ��d����ȴ�ᾧ
��5����֪��NO+NO2+2NaOH=2NaNO2+H2O��
2NO2+2NaOH=NaNO3+NaNO2+H2O
NO��NO2�Ļ���������ɿɱ�ʾΪNOx���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪc
��a��x��1.5 ��b��x=1.2 ��c��X��1.5
��6����֪Ag/��-Al2O3��Ag������������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ������������AgNO3��������
���� ���ڦ�-Al2O3������ϡ���ᣬAg/a-Al2O3�м���ϡ��������ܽ����������������˺���Һ�к���AgNO3������Ϊ��-Al2O3����Һ�м���NaCl�õ�����ΪAgCl���ٹ��˷���õ�AgNO3��NaNO3��Һ��AgClת��ΪAg2O���ܽ���˷��룬���������ܽ�Ag2O�õ�AgNO3������Ũ������ȴ�ᾧ�����˵ȵõ�AgNO3���壮
��1����Ӧ���ɵ������������壬��Ҫ����������������Һ���գ�װ��Ӧ�ܱգ������ܲ�����Һ�����£�
��2������ˮ�к���������������ˮ��Ӧ����HCl��HClO��HCl��AgNO3��Ӧ����AgCl��HNO3��
��3��ʵ�����������Ϊ����������Һ��ӦΪ���ˣ��ݴ���д���貣��������
��4������Һ�л�þ��壬��Ҫ��������Ũ������ȴ�ᾧ�����˵Ȳ�����
��5�����ݷ�Ӧ��NO+NO2+2NaOH�T2NaNO2+H2O��2NO2+2NaOH�TNaNO2+NaNO3+H2O���������ͨ��NaOH��Һ����ȫ����ʱ��Ӧ����n��NO2����n��NO�����ɣ�
��6����֪���������������������������Ļ����ʣ�������֪����������������������������
��� �⣺���ڦ�-Al2O3������ϡ���ᣬAg/a-Al2O3�м���ϡ��������ܽ����������������˺���Һ�к���AgNO3������Ϊ��-Al2O3����Һ�м���NaCl�õ�����ΪAgCl���ٹ��˷���õ�AgNO3��NaNO3��Һ��AgClת��ΪAg2O���ܽ���˷��룬���������ܽ�Ag2O�õ�AgNO3������Ũ������ȴ�ᾧ�����˵ȵõ�AgNO3���壮
��1����Ӧ���ɵ������������壬��Ҫ����������������Һ���գ�װ��b�в��������岻�ܵ������ᵼ��װ����ѹǿ��ǿ������ը�ѵ�Σ�գ�cװ�ò��ܱգ���ѡa��
��2������ˮ�к���������������ˮ��Ӧ����HCl��HClO��HCl��AgNO3��Ӧ����AgCl��HNO3����Ӧ���ӷ���ʽΪ��Ag++Cl-=AgCl����
�ʴ�Ϊ��Ag++Cl-=AgCl����
��3��ʵ�����������Ϊ����������Һ��ӦΪ���ˣ������貣������Ϊ©�����ձ������������ʴ�Ϊ��©�����ձ�����������
��4����AgNO3��Һ�л�þ��壬��Ҫ��������Ũ������ȴ�ᾧ�����˵Ȳ�����˳��Ϊbd��
�ʴ�Ϊ��bd��
��5���ɷ���ʽ��֪�������ͨ��NaOH��Һ����ȫ����ʱ��Ӧ����n��NO2����n��NO������x��1.5����ѡ��c��
��6��Ҫ����Ag�Ļ����ʱ���֪����������������������Ag�������������Ag������������Ҫ֪���������AgNO3���������ʴ�Ϊ��������������AgNO3��������
���� ���⿼���Ʊ������������̡����ʵķ����ᴿ����ԭ���ķ�������ȣ��ؼ�����ȷ����������ÿһ���е��Լ���������ԭ�����Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�| ����ʵ | ���Ʋ� | |
| ��A | Mg��ˮ��Ӧ������Ca��ˮ��Ӧ�Ͽ� | Ba��ˮ��Ӧ����� |
| ��B | ��Si�ǰ뵼����ϣ�ͬ��GeҲ�ǰ뵼����� | ��A���Ԫ�ض��ǰ뵼����ϣ� |
| ��C | ��HCl��1500��ʱ�ֽ⣬HI��230��ʱ�ֽ� | ��HBr�ķֽ��¶Ƚ��ڶ���֮�� |
| ��D | ��Si��H2����ʱ��Ӧ��S��H2�����ܷ�Ӧ | ��P��H2�ڸ���ʱ�ܷ�Ӧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | m=5��n=l | B�� | m=3��n=5 | C�� | m=3��n=4 | D�� | m=3��n=6 |
| A�� | ��ˮ�����硢�������硢���ܷ���ͷ��������У�Ҫ������չ�������� | |
| B�� | �������ϰ�װ����β����ת��װ�ã�ʹ֮�������·�Ӧ��2CO+2NO�T2CO2+N2 | |
| C�� | �ڴ����ƹ��Ҵ����͵�ͬʱ���о�����̫������������ȼ�ϵ������ | |
| D�� | ��úҺ�������������ȼ�ϵ�ȼ��Ч�� |
| t�� | 700 | 800 | 830 | 1 000 | 1 200 |
| K | 1.67 | 1.11 | 1.00 | 0.60 | 0.38 |
| A�� | �÷�Ӧ�Ļ�ѧ����ʽ��CO��g��+H2O��g��?CO2��g��+H2��g�� | |
| B�� | ������Ӧ������Ӧ�Ƿ��ȷ�Ӧ | |
| C�� | ����CO��CO��ƽ��ת�������� | |
| D�� | ��ƽ��Ũ�ȷ��Ϲ�ϵ$\frac{c��C{O}_{2}��}{3c��CO��}$=$\frac{c��{H}_{2}O��}{5c��{H}_{2}��}$�����ʱ���¶�Ϊ1000�� |
| A�� | �������������ڹ��������µ�ȡ����Ӧ | |
| B�� | ��ϩ��ˮ��һ�������µļӳɷ�Ӧ | |
| C�� | �Ҵ��ڿ����м��ȣ���ͭ��Ϊ��������������Ӧ | |
| D�� | ����������������һ�������µļӳɷ�Ӧ |
| A�� | V��N2��=0.2mol•L-1•min-1 | B�� | V��H2��=0.1mol•L-1•min-1 | ||
| C�� | V��NH3��=0.4mol•L-1•min-1 | D�� | V��H2��=0.3mol•L-1•min-1 |
| A�� | �й��ḽ����ˮĤ������Ҫ��ˮ�ı�������Ӱ�� | |
| B�� | ̫��ˮ���д��ڷ��Ӽ������������ | |
| C�� | ��ɫҺ����ȵķ�ɢ��ˮ���У�˵���������ڲ����������˶� | |
| D�� | ��ɫҺ����ȵķ�ɢ��ˮ�������ڻ�ѧ�仯 |
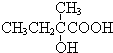
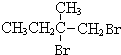 +2NaOH$��_{��}^{ˮ}$
+2NaOH$��_{��}^{ˮ}$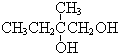 +2NaBr����Ӧ����ȡ����Ӧ
+2NaBr����Ӧ����ȡ����Ӧ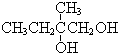 +O2+O2$\stackrel{����}{��}$2
+O2+O2$\stackrel{����}{��}$2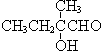 +2H2O����Ӧ����������Ӧ
+2H2O����Ӧ����������Ӧ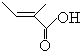 ��
�� ��
��