��Ŀ����
��1�����������ܵ������ ������ţ���ͬ�������ڵ���ʵ��� �����ڷǵ���ʵ��� ��
��ˮ�� ��CuSO4?5H2O �۴���ʯ ���Ȼ��ƾ��� ������ �ް���
������ ������ ��Һ̬�Ȼ��� ��������Һ
��2����Ҫ��ش��������⡣
��Al2��SO4��3�ĵ��뷽��ʽ��
�� NaHCO3�ĵ��뷽��ʽ��
���û�ѧ����ʽ˵��������Ʒ�ĩ�����ܷⱣ���ԭ��
��д��������ˮ��Ӧ�����ӷ���ʽ��
��3����Ҫ�����������գ�
����ͬ���������������顢ˮ�����к������������� ��
��4.9 g H2SO4������ ��ԭ�ӡ�
��a��Xԭ�ӵ�������Ϊb g����X�����ԭ�������ɱ�ʾΪ________��
��ˮ�� ��CuSO4?5H2O �۴���ʯ ���Ȼ��ƾ��� ������ �ް���
������ ������ ��Һ̬�Ȼ��� ��������Һ
��2����Ҫ��ش��������⡣
��Al2��SO4��3�ĵ��뷽��ʽ��
�� NaHCO3�ĵ��뷽��ʽ��
���û�ѧ����ʽ˵��������Ʒ�ĩ�����ܷⱣ���ԭ��
��д��������ˮ��Ӧ�����ӷ���ʽ��
��3����Ҫ�����������գ�
����ͬ���������������顢ˮ�����к������������� ��
��4.9 g H2SO4������ ��ԭ�ӡ�
��a��Xԭ�ӵ�������Ϊb g����X�����ԭ�������ɱ�ʾΪ________��
��1���٢ݢ⣻�ڢۢܢ�ޢ� ��2����Al2��SO4��3=2Al3++3SO42-
��NaHCO3=Na++HCO3- ��Ca(ClO)2+CO2+H2O=CaCO3+2 HClO
(��д��ʽҲ�ɵ�����2HClO 2 HCl+O2��) ��Cl2+H2O
2 HCl+O2��) ��Cl2+H2O  H++Cl-+HClO���á�=�����۷֣�
H++Cl-+HClO���á�=�����۷֣�
��3������������0. 35 NA ����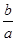 NA��
NA��
��NaHCO3=Na++HCO3- ��Ca(ClO)2+CO2+H2O=CaCO3+2 HClO
(��д��ʽҲ�ɵ�����2HClO
 2 HCl+O2��) ��Cl2+H2O
2 HCl+O2��) ��Cl2+H2O  H++Cl-+HClO���á�=�����۷֣�
H++Cl-+HClO���á�=�����۷֣� ��3������������0. 35 NA ����
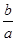 NA��
NA�������������1���٢��ǵ��ʣ����ǽ������ڽ����к��������ƶ��ĵ��ӿ��Ե��磬���Ƿǽ�������û�������ƶ��ĵ��ӻ����ӣ����ܵ��磻�ڢۢ��������ڵ���ʣ������ᣬҲ�ǵ���ʣ����Тڢۢ��京�����ӣ������ڲ��������ƶ����ʲ����Ե��磻��Һ̬�Ȼ����ǹ��ۻ���������� �����ܵ��硣�ݢ��ǻ��������������ƶ������ӣ������磻�ޢ��Ƿǵ���ʣ��������ƶ������ӣ����ܵ��硣 �����������ܵ���������Ǣ٢ݢ⣬���ڵ���ʵ��Ǣڢۢܢᣬ���ڷǵ���ʵ��Ǣޢߡ���2����Al2��SO4��3���Σ�����ǿ����ʣ����ĵ��뷽��ʽ�ǣ�Al2��SO4��3=2Al3++3SO42-�� NaHCO3���������ʽ�Σ�����д���뷽��ʽʱ��������Ӳ��ܲ����ĵ��뷽��ʽ�ǣ�NaHCO3=Na++HCO3-���۴����������ᣬ���Ա�̼�ỹ����������������ڷ��ã���Ϳ����еĶ�����̼��ˮ������Ӧ��Ca(ClO)2+CO2+H2O=CaCO3+2 HClO ������ʧЧ����Ҫ�ܷⱣ�档��������ˮ��Ӧ�����Ȼ��ơ��������ƺ�ˮ�������ӷ���ʽ��Cl2+H2O
 H++Cl-+HClO��3��n=m�MM,n=N�MNA,��ͬ����ʱ���������ʵĵ�Ħ������ԽС�������ʵ�����Խ��������Խ�ࡣ������������������Ħ��������С�����ķ�����Ҳ����ࡣ��������n=m�MM,��������ӵ����ʵ�����������,n=N�MNA���������������ÿ�������к���7��ԭ�ӣ�������������7���͵õ���Ӧ��ԭ��������1��Xԭ�ӵ�����Ϊb�Mag����NA��ԭ�ӵ�������ΪNAb�Mag����Ħ������ΪNAb�Mag�Mmol��X�����ԭ����������ֵ�ϵ�����Ħ��������ΪNAb �Ma��
H++Cl-+HClO��3��n=m�MM,n=N�MNA,��ͬ����ʱ���������ʵĵ�Ħ������ԽС�������ʵ�����Խ��������Խ�ࡣ������������������Ħ��������С�����ķ�����Ҳ����ࡣ��������n=m�MM,��������ӵ����ʵ�����������,n=N�MNA���������������ÿ�������к���7��ԭ�ӣ�������������7���͵õ���Ӧ��ԭ��������1��Xԭ�ӵ�����Ϊb�Mag����NA��ԭ�ӵ�������ΪNAb�Mag����Ħ������ΪNAb�Mag�Mmol��X�����ԭ����������ֵ�ϵ�����Ħ��������ΪNAb �Ma��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
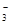 ��SO42-��Fe3+��H+��M�������ʵ���֮��Ϊ��n��NO
��SO42-��Fe3+��H+��M�������ʵ���֮��Ϊ��n��NO ���Un��M����2�U3�U1�U3�U1����M�����������е�
���Un��M����2�U3�U1�U3�U1����M�����������е�