��Ŀ����
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��гּ������������ԣ���
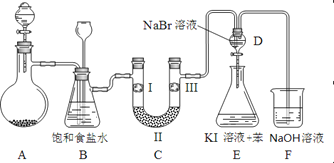
��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾����塾��Ҫ�ɷ�ΪCa��ClO��2����Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ��______________________________________________��
��2��װ��B�б���ʳ��ˮ��������____________��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����_______________________��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η�����________��
a | b | c | d | |
I | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
II | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
III | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ�����Կ�����ɫ��Һ��Ϊ����ɫ��˵���ȵķǽ����Դ����塣��������װ��D��������Һ����װ��E�У����۲쵽��������____________________________��������_______����������������������˵�����������ǿ�ڵ⣬ԭ����___________��
���𰸡� Ca(ClO)2 + 4HCl��Ũ��= CaCl2 + 2Cl2��+ 2H2O ��ȥCl2�е�HCl ��ƿ��Һ���½�������©����Һ������ d E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ ���� ������Cl2Ҳ�ɽ�I������ΪI2 ��
����������1�����������Ũ���ᷴӦ�����Ȼ��ơ�������ˮ����Ӧ����ʽΪCa��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O����2�������ӷ�����Ӧ��ȡ�������к����Ȼ��⣬װ��B�б���ʳ��ˮ�������dz�ȥCl2�е�HCl��װ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������������ʱB�еģ�ѹǿ����B�г���©����Һ���������γ�ˮ������3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư���ԣ�ѡ����abc�Ģ��ж��Ǹ��������ͨ��ʪ�����ɫ����������֤������Ư���ԣ�����C��I��II��III���η���ʪ�����ɫ��������ˮ�Ȼ��ơ��������ɫ����������ѡd����4������D�л���ͨ����������ʱ�����Կ�����ɫ��Һ��Ϊ��ɫ��˵���������廯�Ʒ�Ӧ�����嵥�ʣ�������ӦCl2+2NaBr=Br2+2NaCl����Ӧ������������������ʹ���������������Br2��Cl2����������װ��D�к��嵥�ʵ�������Һ�����ʵ⻯�غͱ���װ��E�У��嵥�ʺ͵⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ�����ڱ����Ϻ�ɫ�������۲쵽�������ǣ�E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ��
���磺���⿼��������ʵ������ȡ��������ѧ���ʡ�ʵ����ơ�ʵ��װ�õ��������ۡ���ѧ����ʽ����д�ȣ���Ŀ�Ѷ��еȣ��Ƕ���ѧ֪ʶ���ۺ����ã�ע�����֪ʶ�����ա�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����м���Ԫ�ص����ʻ�ԭ�ӽṹ���±���
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
T | ʧȥһ�����Ӻ��γ�Neԭ�ӵ��Ӳ�ṹ |
X | �����������Ǵ�����������2�� |
Y | �䵥��֮һ�ǿ�������Ҫ�ɷ֣��������ȼ�� |
Z | �γ�˫ԭ�ӵ��ʷ��ӣ�����ɫ���� |
��1��Ԫ��X��һ��ͬλ��������ԭ������������ͬλ�صķ�����__________��
��2������T��ԭ�ӽṹʾ��ͼ__________��
��3��д����T��Y��Z����Ԫ����ɵ�һ�ֻ�������ˮ��Һ�еĵ��뷽��ʽ__________��
����Ŀ��ijѧϰС�鰴����ʵ������̽�������е⺬���IJⶨ�͵����ȡ��
ʵ��(һ) �⺬���IJⶨ
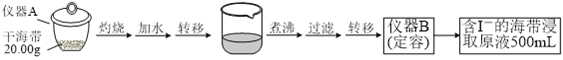
ȡ0.0100 mol��L��1��AgNO3����Һװ��ζ��ܣ�ȡ100.00 mL������ȡԭҺ���ζ��أ��õ��Ƶζ����ⶨ�⺬������õĵ綯��(E) ��ӳ��Һ��c(I��)�ı仯�������������±���
V(AgNO3)/mL | 15.00 | 19.00 | 19.80 | 19.98 | 20.00 | 20.02 | 21.00 | 23.00 | 25.00 |
E/mV | ��225 | ��200 | ��150 | ��100 | 50.0 | 175 | 275 | 300 | 325 |
ʵ��(��) �����ȡ
���ƺ�����ȡԭҺ���ס�������ʵ�鷽�����£�
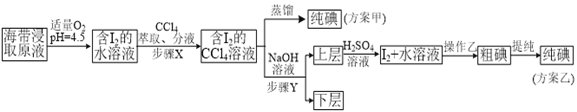
��֪��3I2��6NaOH��5NaI��NaIO3��3H2O��
��ش�
��1��ʵ��(һ) �е��������ƣ�����A_________�� ���� B___________________��
��2���ôεζ��յ�ʱ��ȥAgNO3��Һ�����Ϊ20.00mL������ú����е�İٷֺ���Ϊ_______%��
��3���ٷ�Һ©��ʹ��ǰ���©����©����Ϊ___________________��
�ڲ���X�У���ȡ���Һ©���ڹ۲쵽��������_______________��
�������йز���Y��˵������ȷ����___________________��
A.Ӧ����NaOH��Һ��Ũ�Ⱥ���� B.����ת�������ӽ���ˮ��
C.��Ҫ�dz�ȥ������ȡԭҺ�е��л����� D.NaOH��Һ�������Ҵ�����
��ʵ��(��) �в���Z��������______________________��
��4�������������������������_____________________��