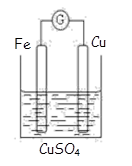
��Ŀ����
CuI��һ�ֲ�����ˮ�İ�ɫ���壬�����ɷ�Ӧ��2Cu2��+4I�� =2CuI��+I2���õ�������ͭƬ��ʯī���缫�����KI��Һ��ȡCuI��Ϊȷ�Ϸ�Ӧ�����ͨ��ǰ����Һ���ּ�����������̪��Һ�͵�����Һ�����һ��ʱ���õ���ɫ������ͬʱ��������Һ��죬��������Һ����������˵����ȷ����
A��ͭƬ��������ʯī������
B����ɫ������������������
C����������Һ������ԭ���ǣ�2Cu+4I����4e��=2CuI��+I2���������۱���
D����������Һ������ԭ���ǣ�4OH����4e��=2H2O+O2����O2��I������ΪI2���������۱���
���𰸡�
C
��������

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�����Ŀ
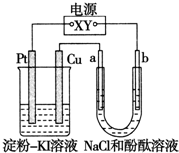 ��2011?�㽭��У����������CuI��һ�ֲ�����ˮ�İ�ɫ���壬�������ɷ�Ӧ��2Cu2++4I-�T2CuI��+I2���õ�����ͼ��ʾװ���У�a��b���Ƕ��Ե缫��ͨ��һ��ʱ����ڵ���-KI��Һ��������Χ����ɫ��������˵����ȷ���ǣ�������
��2011?�㽭��У����������CuI��һ�ֲ�����ˮ�İ�ɫ���壬�������ɷ�Ӧ��2Cu2++4I-�T2CuI��+I2���õ�����ͼ��ʾװ���У�a��b���Ƕ��Ե缫��ͨ��һ��ʱ����ڵ���-KI��Һ��������Χ����ɫ��������˵����ȷ���ǣ�������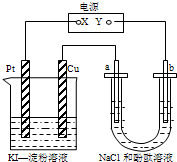 CuI��һ�ֲ�����ˮ�İ�ɫ���壬�������ɷ�Ӧ��2Cu2++4I-=2CuI��+I2���õ�����ͼ��ʾװ���У�a��b���Ƕ��Ե缫��ͨ��һ��ʱ�����KI-������Һ��������Χ����ɫ��������˵����ȷ���ǣ�������
CuI��һ�ֲ�����ˮ�İ�ɫ���壬�������ɷ�Ӧ��2Cu2++4I-=2CuI��+I2���õ�����ͼ��ʾװ���У�a��b���Ƕ��Ե缫��ͨ��һ��ʱ�����KI-������Һ��������Χ����ɫ��������˵����ȷ���ǣ�������