��Ŀ����
��8�֣�(1)��ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ�������������Ӱ�ˮ���������ܽ�õ�����ɫ������Һ������˵����ȷ����
(2)д��(1)��Ӧ���̵����ӷ���ʽ�� ��
��(1)�õ�����Һ�м����Ҵ�����������ɫ�ľ����仯ѧʽ
| A����Ӧ����Һ�в������κγ��������Է�Ӧǰ��Cu2+��Ũ�Ȳ��� |
| B�������ܽ����������ɫ���������[Cu��NH3��4] 2+ |
| C��[Cu��NH3��4] 2+����������ΪCu2+ |
| D����[Cu��NH3��4] 2+�����У�Cu2+�����¶Ե��ӣ�NH3�е�Nԭ���ṩ�չ�� |
��(1)�õ�����Һ�м����Ҵ�����������ɫ�ľ����仯ѧʽ
��ÿ��2�֣���8�֣���1��B��C ��2��Cu2++2NH3.H2O = Cu(OH) 2��+2 NH4+
Cu(OH) 2+4 NH3 =" [Cu" (NH3) 4]2+ + 2OH- [Cu (NH3) 4] SO4 .H2O
Cu(OH) 2+4 NH3 =" [Cu" (NH3) 4]2+ + 2OH- [Cu (NH3) 4] SO4 .H2O
����ͭ����ˮ��Ӧ����������ͭ��ɫ�������������백ˮ��������ͭ�ܽ��ڰ�ˮ���γ��������а��������壬�ṩ�¶Ե��ӣ�ͭ�����ṩ�չ����������ȷ�Ĵ���BC��Ӧ�÷�ӦʽΪCu2++2NH3.H2O = Cu(OH) 2��+2 NH4+��u(OH) 2+4 NH3 =" [Cu" (NH3) 4]2+ + 2OH-�������Ҵ��ļ��Խ�С�����Ի��γɾ��壬�仯ѧʽΪ[Cu (NH3) 4] SO4 .H2O��

��ϰ��ϵ�д�
�����Ŀ
 O2[PtF6]��O2[PtF6]Ϊ��λ���������PtΪ��5�ۣ������ڴ˷�Ӧ������˵����ȷ����
O2[PtF6]��O2[PtF6]Ϊ��λ���������PtΪ��5�ۣ������ڴ˷�Ӧ������˵����ȷ����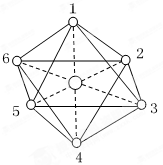

 ��
��