��Ŀ����
��֪��C(s)+O2(g)===CO2(g) ��H=-393��5 kJ��mol-1��CO(g)+1/2O2(g) ===CO2(g) ��H=-283 kJ��mol-1����
C(s)��O2(g)��Ӧ����1mol CO(g)�ķ�Ӧ��Ϊ
C(s)��O2(g)��Ӧ����1mol CO(g)�ķ�Ӧ��Ϊ
[ ]
A����H=-676��5 kJ��mol-1
B����H=+676��5 kJ��mol-1
C����H=-110��5 kJ��mol-1
D����H=+110��5 kJ��mol-1
B����H=+676��5 kJ��mol-1
C����H=-110��5 kJ��mol-1
D����H=+110��5 kJ��mol-1
C

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ

 CO2(g) ��H����437.3 kJ?mol��1
CO2(g) ��H����437.3 kJ?mol��1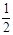 O2(g)
O2(g)  CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��