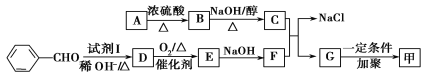
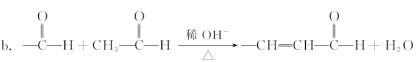
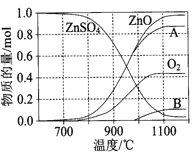
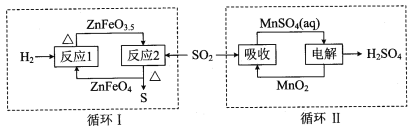
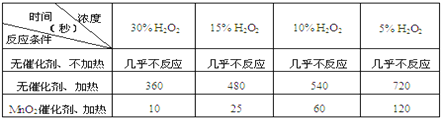
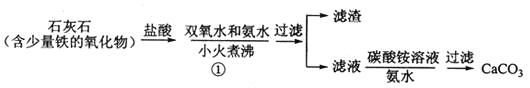
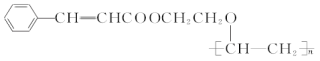��Ŀ����
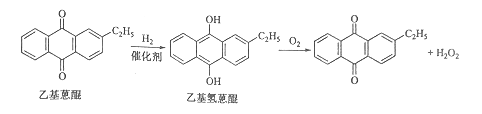
����Ŀ��[��ѧ-ѡ��2����ѧ�뼼��]˫��ˮ��һ����Ҫ����������Ư����������������˫��ˮ���������������䷴Ӧԭ��������������ͼ��ʾ��
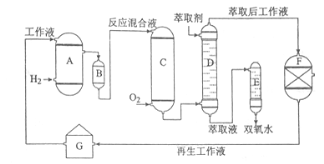
A���⻯��B��������C��������D����ȡ��E.������F.����Һ����װ��G.����Һ����װ��
���������У����һ����������л��ܼ����Ƴɹ���Һ����һ�����¶ȡ�ѹ���ʹ��������½����⻯���پ���������ȡ�������ȹ��յõ�˫��ˮ���ش��������⣺
��1���������Ʊ�˫��ˮ���������ĵ�ԭ����_______��ѭ��ʹ�õ�|ԭ����______�����ƹ���Һʱ�����л��ܼ���������ˮ��ԭ����______��
��2���⻯��A�з�Ӧ�Ļ�ѧ����ʽΪ_______������������C�ķ�Ӧ���Һ�е���Ҫ����Ϊ_______��
��3����ȡ��D�е���ȡ����____��ѡ��������ȡ����ԭ����______��
��4������Һ����װ��F��Ҫ����������H2O2��ԭ����______��
��5��˫��ˮŨ�ȿ���������������KMnO4��Һ�ⶨ���÷�Ӧ�����ӷ���ʽΪ_______��һ��˫��ˮ����������Ϊ27.5%�����ܶ�Ϊ1.10g��cm3������Ũ��Ϊ______mol/L��
���𰸡���1�������������һ������һ��������һ���������������ˮ���������л��ܼ�
��2��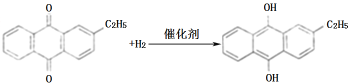 �һ�������
�һ�������
��3��ˮ H2O2����ˮ��ˮ��ȡ���һ�����������ˮ
��4��H2O2�ֽ�ų���������������ϣ�������ը
��5��6H++5H2O2+2MnO4-=2Mn2++5O2��+8H2O ��8.9
��������
�����������1�����ݷ�Ӧԭ����֪���������Ʊ�˫��ˮ���������ĵ�ԭ�����������������ɹ�������ͼ��֪��ѭ��ʹ�õ�ԭ�����һ��������һ����������л��������������ԭ�����һ��������һ���������������ˮ���������л��ܼ����������ƹ���Һʱ�����л��ܼ���������ˮ��
��2�����ݷ�Ӧԭ�����⻯��A�з�Ӧ�Ļ�ѧ����ʽΪ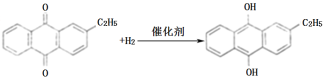 ������������C�ķ�Ӧ���Һ�е���Ҫ����Ϊ�һ���������
������������C�ķ�Ӧ���Һ�е���Ҫ����Ϊ�һ���������
��3����ȡ��D����Ҫ����˫��ˮ���һ�������H2O2����ˮ��ˮ��ȡ���һ�����������ˮ������ѡȡ����ȡ����ˮ��
��4��H2O2�ֽ�ų���������������ϣ�������ը���������Һ����װ��F��Ҫ����������H2O2��
��5��˫��ˮ��������������KMnO4����������ԭ��Ӧ��MnԪ�صĻ��ϼ���+7�۽��͵�+2�ۣ�OԪ�صĻ��ϼ���-1�����ߵ�0�ۣ����ݵ�ʧ�����غ㡢����غ��ԭ���غ���ƽ���÷�Ӧ�����ӷ���ʽΪ6H++5H2O2+2MnO4-=2Mn2++5O2��+8H2O��c=1000��1.10��27.5%��34=8.9mol/L��