��Ŀ����
��ⷨ�ٽ����ʯ����Ҫ�ɷ���Mg2SiO4���̶�CO2�IJ��ֹ����������£�
��֪��Mg2SiO4(s) +4HCl(aq) 2MgCl2(aq) +SiO2 (s) + 2H2O(l) ��H =��49.04 kJ��mol-1
2MgCl2(aq) +SiO2 (s) + 2H2O(l) ��H =��49.04 kJ��mol-1
��1��ij���ʯ�������Mg9FeSi5O20�������������ʽ�ɱ�ʾΪ ��
��2����̼ʱ��Ҫ��Ӧ�ķ���ʽΪNaOH(aq)+CO2 (g)=NaHCO3 (aq)���÷�Ӧ���Է����е�ԭ���� ��
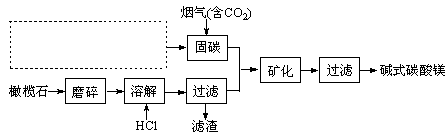
��3��������ͼ����ڲ���һ����ҵ�������� ��
��4������������Ҳ����������̼������ ��������ĸ��
a��CaCl2 b��H2NCH2COONa c��(NH4)2CO3
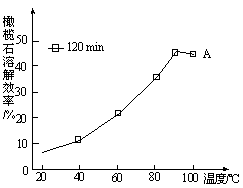
��5����ͼ��֪��90�������A�ܽ�Ч���½���������ԭ�� ��
��6�������������ü�ʽ̼��þ��Ʒ�к�������NaCl��Fe2O3��Ϊ�ᴿ���ɲ�ȡ�Ĵ�ʩ����Ϊ�����ܽ��������Һ���г����������Բ�Ʒ����ϴ�Ӵ������жϲ�Ʒϴ���IJ����� ��
��֪��Mg2SiO4(s) +4HCl(aq)
 2MgCl2(aq) +SiO2 (s) + 2H2O(l) ��H =��49.04 kJ��mol-1
2MgCl2(aq) +SiO2 (s) + 2H2O(l) ��H =��49.04 kJ��mol-1
��1��ij���ʯ�������Mg9FeSi5O20�������������ʽ�ɱ�ʾΪ ��
��2����̼ʱ��Ҫ��Ӧ�ķ���ʽΪNaOH(aq)+CO2 (g)=NaHCO3 (aq)���÷�Ӧ���Է����е�ԭ���� ��
��3��������ͼ����ڲ���һ����ҵ�������� ��
��4������������Ҳ����������̼������ ��������ĸ��
a��CaCl2 b��H2NCH2COONa c��(NH4)2CO3
��5����ͼ��֪��90�������A�ܽ�Ч���½���������ԭ�� ��

��6�������������ü�ʽ̼��þ��Ʒ�к�������NaCl��Fe2O3��Ϊ�ᴿ���ɲ�ȡ�Ĵ�ʩ����Ϊ�����ܽ��������Һ���г����������Բ�Ʒ����ϴ�Ӵ������жϲ�Ʒϴ���IJ����� ��
��1��9MgO��FeO��5SiO2 ��2����H��0
��3�� ��
��
��4��bc
��5��120min���ܽ�ﵽƽ�⣬����Ӧ���ȣ�����ƽ�������ƶ����ܽ�Ч�ʽ��͡�
��6��ȡ�������һ�ε�ϴ��Һ���������ữ����������Һ������������������ϴ����
��3��
 ��
��
��4��bc
��5��120min���ܽ�ﵽƽ�⣬����Ӧ���ȣ�����ƽ�������ƶ����ܽ�Ч�ʽ��͡�
��6��ȡ�������һ�ε�ϴ��Һ���������ữ����������Һ������������������ϴ����
�����������1������������ʽ��ʾ�����Ρ��������к�����Ԫ�ء�Si��O���еĻ��нᾧˮ����������д�����������дSiO2��Ҫ����ˮ��H2Oд�����Ҫ���ж��ֽ���Ԫ�أ������˳��������ý�����������ǰ�������ý����������ں����ʯ�������Mg9FeSi5O20�������������ʽ�ɱ�ʾΪ9MgO��FeO��5SiO2����2�����Է����еķ�Ӧ���������ƣ�һ������Ӧ�Ƿ��ȷ�Ӧ����һ�ǻ��Ҷ���������NaOH(aq)+CO2 (g)=NaHCO3 (aq)���÷�Ӧ�ǻ��Ҷȼ�С���������Է���Ҫ�Է�ֻ��������Ӧ�Ƿ��ȷ�Ӧ������H��0 ����3���ӹ�̼�ķ�Ӧ���Կ�����Ҫ�õ�����������Һ������ͼ����ڲ���õ���������һ����ҵ�������̼��ɣ�����ͨ����ⱥ�͵��Ȼ�����Һ������дΪ
 ��
��
����4��a��CaCl2�����������̼��Ӧ�� b��H2NCH2COONa �ǰ����ᣬ���������ᷴӦ�����Σ��ܹ�̼�� c��(NH4)2CO3��Һ���������̼��Ӧ������̼����李���ѡbc����5����ͼ��֪��120min���ܽ�ﵽƽ�⣬����Ӧ���ȣ�����ƽ�������ƶ����ܽ�Ч�ʽ��͡���6���²�Ʒ��û��ϴ����������Cl-���жϲ�Ʒϴ���IJ����ǿ���ͨ�����������Ӽ��ɣ�������ȡ�������һ�ε�ϴ��Һ���������ữ����������Һ������������������ϴ����

��ϰ��ϵ�д�
 ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д�
�����Ŀ
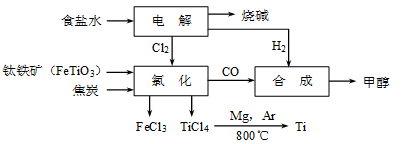
 CH3OH(g)�����������������������ʵ��κ���ʧ��������ҵ����ÿ�ϳ�6mol�״�����������ⲹ��H2 mol��
CH3OH(g)�����������������������ʵ��κ���ʧ��������ҵ����ÿ�ϳ�6mol�״�����������ⲹ��H2 mol�� 4Ag+O2��
4Ag+O2�� 2Fe+3CO2��
2Fe+3CO2�� Mg+C12��
Mg+C12��