��Ŀ����
����Ŀ�����������[(NH4)2SO4��FeSO4��6H2O]�׳�Ī���Σ�������ˮ����100����110��ʱ�ֽ⡣�ڶ��������г������궨�ظ���ء�������ص���Һ�ı����ʣ�������ұ�𡢵�Ƶȡ�
��.��ѧ����С���о�Ī���ξ���ǿ��ʱ�ķֽ������
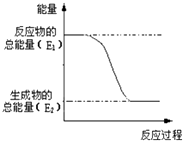
(1)����ͬѧ������ͼ��ʾ��װ�ý����о���װ��C�пɹ۲쵽��������___________���ɴ˿�֪�ֽ��������_____________��

(2)����ͬѧ��ΪĪ���ξ���ֽ�IJ����л�����SO3(g)��SO2(g)��N2��Ϊ������֤��ѡ�ü���ʵ���е�װ��A����ͼ��ʾ�IJ���װ�ý���ʵ����

������ͬѧ��ʵ���У�װ���������ӵĺ���˳��ΪA��__________________��
��װ��D�����������������____________������SO3��ʵ��������_______________��
��.Ϊ����������林��ȣ���ȡm gĪ������Ʒ�����500mL��Һ���ס��ҡ�����λͬѧ�������������ʵ�鷽������ش�
������ȡ25.00mL�����������Һ��0.1000mol��L-1������KMnO4��Һ�����ν��еζ���
�ҷ�����ȡ25.00mL�����������Һ��������ʵ����

����������ͨ��NH4+�ⶨ��ʵ�����ͼ����ͼ��ʾ��ȡ25.00mL�����������Һ���и�ʵ����

(3)�����е����ӷ���ʽΪ______________________����ʵ���������ȷ������ⶨ�������С���ҷ���������ԭ��Ϊ________________����֤�Ʋ�ķ���Ϊ___________________________________ ��
(4)�ҷ����г����Ƿ�ϴ�Ӹɾ��ļ��鷽����______________________________��
(5)��������������������Լ���__________________
a.ˮ b.����NaHCO3��Һ c.CCl4
(6)�����NH3�����������Ϊ��״���£�ΪV L������������林���Ϊ_________________��
���𰸡� ��Һ��� NH3 G D B C F ���հ���������Һ�ữ���ų�SO2�ĸ��� D�г��ְ�ɫ���� MnO4-+5Fe2+ 8H+ =Mn2++5Fe3+ + 4H2O Fe2+�ѱ������������� ȡ���������������Һ����������KSCN��Һ������Һ��ΪѪ��ɫ��˵��Fe2+���������������� ȡ���һ��ϴ�ӵ���Һ����AgNO3��Һ����û�г�����˵����ϴ�ɾ� c 175V/m ��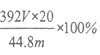
������������(1)Ī����[(NH4)2Fe(SO4)26H2O]���ȷֽ⣬�ֽ�ʱ����������Ͷ�����������������������壬����װ��ͼ��֪����ʯ�ҿ��������������壬����������̪��Һ����ɫ������װ��C�пɹ۲쵽����������Һ��죬�ɴ˿�֪Ī���ξ���ֽ�IJ������� NH3��װ��B����Ҫ������ ���շֽ�������������壬�ʴ�Ϊ����Һ��죻NH3��
(2)��Ҫ��������SO3(g)��SO2(g)��N2���ڼ���ʵ���е�װ��A�������������ȫƿ��ͨ���Ȼ�����Һ����SO3����ͨ��Ʒ����Һ����SO2����Ũ�������Ƴ�ȥ������������ˮ�������ռ�����������װ���������ӵĺ���˳��ΪA��G��D��B��C��F���ʴ�Ϊ��G��D��B��C��F��
�����ڲ������������а����������Ȼ�����Һ�м������������ᣬ�������հ�������ֹ���������ᱵ�������ų�SO2�ĸ��ţ�SO3ͨ���Ȼ�����Һ�п��Բ������ᱵ����������������ʹƷ����ɫ������װ��D����������������� ���հ���������Һ�ữ���ų�SO2�ĸ��ţ�����SO3��ʵ�������� D���а�ɫ�������ʴ�Ϊ�����հ���������Һ�ữ���ų�SO2�ĸ��ţ�D���а�ɫ������
����(3)���������Һ���������ԣ���������������Ϊ�����ӣ���Ӧ�����ӷ���ʽΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O���������Ӿ��л�ԭ�ԣ��ױ������������ӣ��ʲ�����������Ũ��ƫС���ɼ����������ӵ��������������ӣ��������Ϊ��ȡ���������������Һ����������KSCN��Һ������Һ��ΪѪ��ɫ��˵��Fe2+�ѱ����������������ʴ�Ϊ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��Fe2+�ѱ���������������ȡ���������������Һ����������KSCN��Һ������Һ��ΪѪ��ɫ��˵��Fe2+�ѱ���������������
(4)�ҷ����г����Ƿ�ϴ�Ӹɾ����������ʵ�����ϴ��Һ���Ƿ��������Ӽ������ϴ���Ƿ�ɾ������岽��Ϊ��ȡ���һ��ϴ�ӵ���Һ����AgNO3��Һ����û�г�����˵����ϴ�ɾ����ʴ�Ϊ��ȡ���һ��ϴ�ӵ���Һ����AgNO3��Һ����û�г�����˵����ϴ�ɾ���
(5)��װ���е�����Һ�����ϣ�������Һ���ռ�����Ҫ����������Һ��Ӧ�����ܽⰱ��������������ˮ�ͱ���̼������Һ�����������Ȼ�̼�����������Ȼ�̼���ռ����ʴ�Ϊ��c��
(6)VL���������ʵ���Ϊ�� ![]() =
=![]() mol��m g�����������Ʒ�к�N�����ʵ���Ϊ
mol��m g�����������Ʒ�к�N�����ʵ���Ϊ![]() ��
��![]() mol=
mol=![]() mol����������淋Ĵ���Ϊ��
mol����������淋Ĵ���Ϊ�� 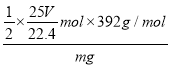 ��100%=
��100%=![]() ��100%���ʴ�Ϊ��
��100%���ʴ�Ϊ�� ![]() ��100%��
��100%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�