��Ŀ����
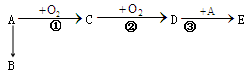
��11�֣��л���A��C5H10O���ܹ�������ͼ��ʾ����ط�Ӧ����֪A�����к���2��λ�ò�ͬ�ļ���E����������

��1��A��������__________________����ķ�Ӧ������__________________��
��2���Լ�a�����_______________________________��
��3��A��һ��ͬ���칹��ĺ˴Ź�����������4���壨���ֲ�ͬλ�õ���ԭ�ӣ�����ͬ���칹��Ľṹ��ʽΪ ___________________________��
��4��һ�������£�B��C��Ӧ��������ζ���л������д���÷�Ӧ�Ļ�ѧ����ʽ��_______________��
��5����B������ͬ�����ŵ�ͬ���칹��(������B)�� __________________ �֡�

��1��A��������__________________����ķ�Ӧ������__________________��
��2���Լ�a�����_______________________________��
��3��A��һ��ͬ���칹��ĺ˴Ź�����������4���壨���ֲ�ͬλ�õ���ԭ�ӣ�����ͬ���칹��Ľṹ��ʽΪ ___________________________��
��4��һ�������£�B��C��Ӧ��������ζ���л������д���÷�Ӧ�Ļ�ѧ����ʽ��_______________��
��5����B������ͬ�����ŵ�ͬ���칹��(������B)�� __________________ �֡�
��1��2������1����ȩ����2��������ȩ����ȡ����Ӧ����2��NaOH���Ҵ���Һ��
��3��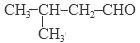 ����3������2����ϩ����2����ͪҲ�ɣ���
����3������2����ϩ����2����ͪҲ�ɣ���
��4��CH3CH2CH(CH3)COOH+CH3CH2CH(CH3)CH2OH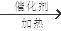
 +H2O
+H2O
��5��3��
��3��
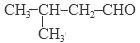 ����3������2����ϩ����2����ͪҲ�ɣ���
����3������2����ϩ����2����ͪҲ�ɣ�����4��CH3CH2CH(CH3)COOH+CH3CH2CH(CH3)CH2OH
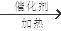
 +H2O
+H2O��5��3��
�����������1���л���A����ʽ��C5H10O������ȩ��ͪ��ͨʽ������A�����к���2��λ�ò�ͬ�ļ�����������ͼ�������ת����ϵ����AΪCH3CH2CH(CH3)CHO������Ϊ2��������ȩ����2��A��H2�����ӳɷ�Ӧ�õ�C��CH3CH2CH(CH3)CH2OH��C��HBr�ڼ���ʱ����ȡ����Ӧ�õ�D��CH3CH2CH(CH3)CH2Br CH3CH2CH(CH3)CH2OH+HBr
 CH3CH2CH(CH3)CH2Br+H2O��D��NaOH���Ҵ���Һ���ȣ�������ȥ��Ӧ�õ�E��
CH3CH2CH(CH3)CH2Br+H2O��D��NaOH���Ҵ���Һ���ȣ�������ȥ��Ӧ�õ�E�� ��HBr��C��CH3CH2CH(CH3)CH2OH��Ũ�����ϼ���Ҳ�ɷ�����ȥ��Ӧ�õ�E��
��HBr��C��CH3CH2CH(CH3)CH2OH��Ũ�����ϼ���Ҳ�ɷ�����ȥ��Ӧ�õ�E�� ��ˮ����3��A��һ��ͬ���칹��ĺ˴Ź�����������4���壨���ֲ�ͬλ�õ���ԭ�ӣ�����ͬ���칹��Ľṹ��ʽΪ
��ˮ����3��A��һ��ͬ���칹��ĺ˴Ź�����������4���壨���ֲ�ͬλ�õ���ԭ�ӣ�����ͬ���칹��Ľṹ��ʽΪ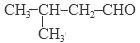 ����4��������һ�������£�B��C��Ӧ��������ζ���л������C�Ǵ�CH3CH2CH(CH3)CH2OH����A������Ӧ������BӦ��Ϊ�ᡣ��CH3CH2CH(CH3)COOH���÷�Ӧ�Ļ�ѧ����ʽΪCH3CH2CH(CH3)COOH+CH3CH2CH(CH3)CH2OH
����4��������һ�������£�B��C��Ӧ��������ζ���л������C�Ǵ�CH3CH2CH(CH3)CH2OH����A������Ӧ������BӦ��Ϊ�ᡣ��CH3CH2CH(CH3)COOH���÷�Ӧ�Ļ�ѧ����ʽΪCH3CH2CH(CH3)COOH+CH3CH2CH(CH3)CH2OH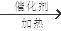
 +H2O����5����B������ͬ�����ŵ�ͬ���칹����CH3CH2CH2CH2COOH��
+H2O����5����B������ͬ�����ŵ�ͬ���칹����CH3CH2CH2CH2COOH��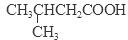 ��
�� �����֡�
�����֡�
��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
�����Ŀ
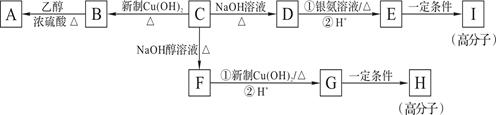
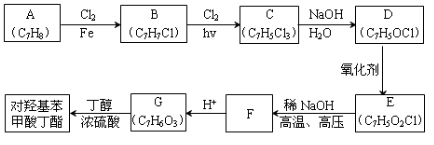
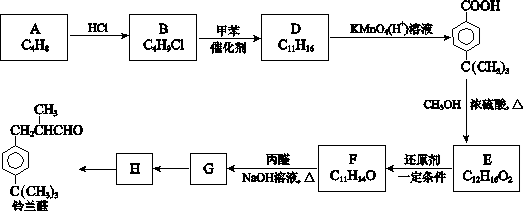
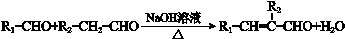

 ���ϳ�·���� ��
���ϳ�·���� ��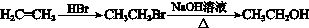

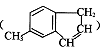 ��һ��ҽҩ�ϳ��м��壬ijͬѧ������ĺϳ�·�����£�
��һ��ҽҩ�ϳ��м��壬ijͬѧ������ĺϳ�·�����£�
 ����˵����ȷ���� ������ţ���
����˵����ȷ���� ������ţ���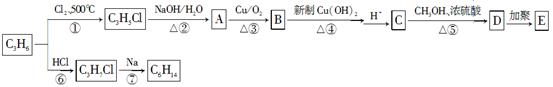
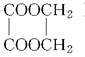 )����ȷ˳����(����)
)����ȷ˳����(����)