��Ŀ����
����Ŀ��A��B��C��D 4��Ԫ�أ�AԪ��������������������������ԭ����������ȣ�B��ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ������2����C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��
��1��BԪ�ص�����Ϊ��
��2��A��B�γɵĻ�����ĵ���ʽΪ ��
��3��C��Ԫ�ط���Ϊ �� C�����������Ļ�ѧʽΪ ��
��4��D������������Ӧ��ˮ����Ļ�ѧʽΪ ��
���𰸡�
��1����
��2��![]()
��3��S��H2SO4
��4��KOH
���������⣺A��B��C��D 4��Ԫ�أ�AԪ��������������������������ԭ����������ȣ���AΪHԪ�أ�B��ԭ�Ӱ뾶����������������С�ģ����ڵڶ����ڣ�B������������Ӧˮ����Ļ�ѧʽΪHBO3 �� ��������ϼ�Ϊ+5�����ڢ�A�壬��BΪNԪ�أ�CԪ��ԭ�ӵ������������ȴ������2������CӦ���������Ӳ㣬����������Ϊ6����CΪSԪ�أ�C����������D�������Ӿ�����ͬ�ĵ����Ų������Ӻ��������Ϊ18����Ԫ�ؿ��γɻ�����D2C��D�ڵ������ڵڢ�A�壬��DΪK����1��BΪNԪ�أ�Ԫ�ص�����Ϊ�������Դ��ǣ�������2��A��B�γɵĻ�����ΪNH3 �� �����ʽΪ ![]() �����Դ��ǣ�
�����Դ��ǣ� ![]() ����3��C��Ԫ�ط���ΪS��C�����������Ļ�ѧʽΪH2SO4 �� ���Դ��ǣ�S��H2SO4����4��D������������Ӧ��ˮ����ΪKOH�����Դ��ǣ�KOH��
����3��C��Ԫ�ط���ΪS��C�����������Ļ�ѧʽΪH2SO4 �� ���Դ��ǣ�S��H2SO4����4��D������������Ӧ��ˮ����ΪKOH�����Դ��ǣ�KOH��

 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д�����Ŀ����п�����ᷴӦ��ʵ���У�һ��ѧ���õ��Ľ�����±���ʾ��
��� | п������/g | п����״ | �¶�/�� | ��ȫ�ܽ� |
A | 2 | ��Ƭ | 5 | 400 |
B | 2 | ��Ƭ | 15 | 200 |
C | 2 | ��Ƭ | 25 | 100 |
D | 2 | ��Ƭ | 35 | t1 |
E | 2 | ϸС���� | 15 | t2 |
F | 2 | ��ĩ | 15 | t3 |
G | 2 | ��Ƭ������������Cu�� | 35 | t4 |
��1��t1=s��������ʱ����¶ȵ�����ͼ�������ʾʱ�䣬�����ʾ�¶ȣ��� 
��2����������������ͼ���ܽᲢ�ó��Ĺ����¶�Ӱ�췴Ӧ���ʵĽ�������
��3��t1t4���������������ԭ���� �� t2t3���������������ԭ������
��4����λʱ��������п������mB��mE��mF�Ӵ�С��˳��Ϊ ��
����Ŀ���ؽ���Ԫ�ظ��Ķ��Խϴ�����ˮ�辭������������ŷš�
������ij��ҵ��ˮ����Ҫ����Cr3+��ͬʱ������������Fe2+��Fe3+��Al3+��Ca2+��Mg2+�ȣ������Խ�ǿ��Ϊ�������ã�ͨ�������������̴�����
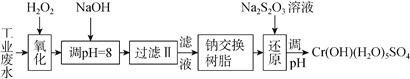
ע�����������ӳ�����������������ʽ��ȫ����ʱ��Һ��pH���±���
�������� | Fe(OH)3 | Fe(OH)2 | Mg(OH)2 | Al(OH)3 | Cr(OH)3 |
pH | 3.7 | 9.6 | 11.1 | 8 | 9(��9�ܽ�) |
��1�����������пɴ���H2O2������Լ���________�����������
A.Na2O2����B.HNO3����C.FeCl3����D.KMnO4
��2������NaOH��Һ������ҺpH=8ʱ����ȥ��������___�������������֪�����ӽ�����֬��ԭ����Mn++nNaRMRn+nNa+���˲�������������ȥ����������___�����������
A.Fe3+����B.Al3+����C.Ca2+����D.Mg2+
��3���ڻ�ԭ�����У�ÿ����172.8gCr2O72- ת��4.8mole-����ԭ�����и÷�Ӧ���ӷ���ʽΪ____������֪������������Cr3+ת��ΪCr2O72- ��
���������������£���Ԫ����Ҫ��Cr2O72-��ʽ���ڣ���ҵ�ϳ��õ�ⷨ������Cr2O72-�ķ�ˮ��ʵ����������ͼװ��ģ����÷�ˮ��������Ӧ��Fe-2e-![]() Fe2+��������Ӧʽ��2H++2e-
Fe2+��������Ӧʽ��2H++2e-![]() H2����
H2����
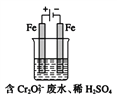
��1�����ʱ�ܷ���Cu�缫������Fe�缫��______������������������������������_____��
��2�����ʱ����������Һ��ת��ΪCr3+�����ӷ���ʽΪ___________________��
��3��������Ӧ�õ��Ľ������������������ɳ�����ȫ�������ˮ�ĵ���ƽ��Ӱ��ǶȽ�����ԭ��______________________��
��4������Һ�г�ʼ����0.1mol Cr2O72-�������ɵ�������ȫ��ת���ɳ�����������_______g��
